Pulmonary alveolar echinococcosis (PAE) is a chronic disease caused by Echinococcus multilocularis with very low incidence in developed countries.
MethodsThis single-center, retrospective study included 34 patients who were diagnosed with PAE between January 2001 and February 2019 (15 males, 19 females, mean age: 52.4±15.8 years, age range: 28–78 years) in Ataturk University Medical School, Erzurum, Turkey.
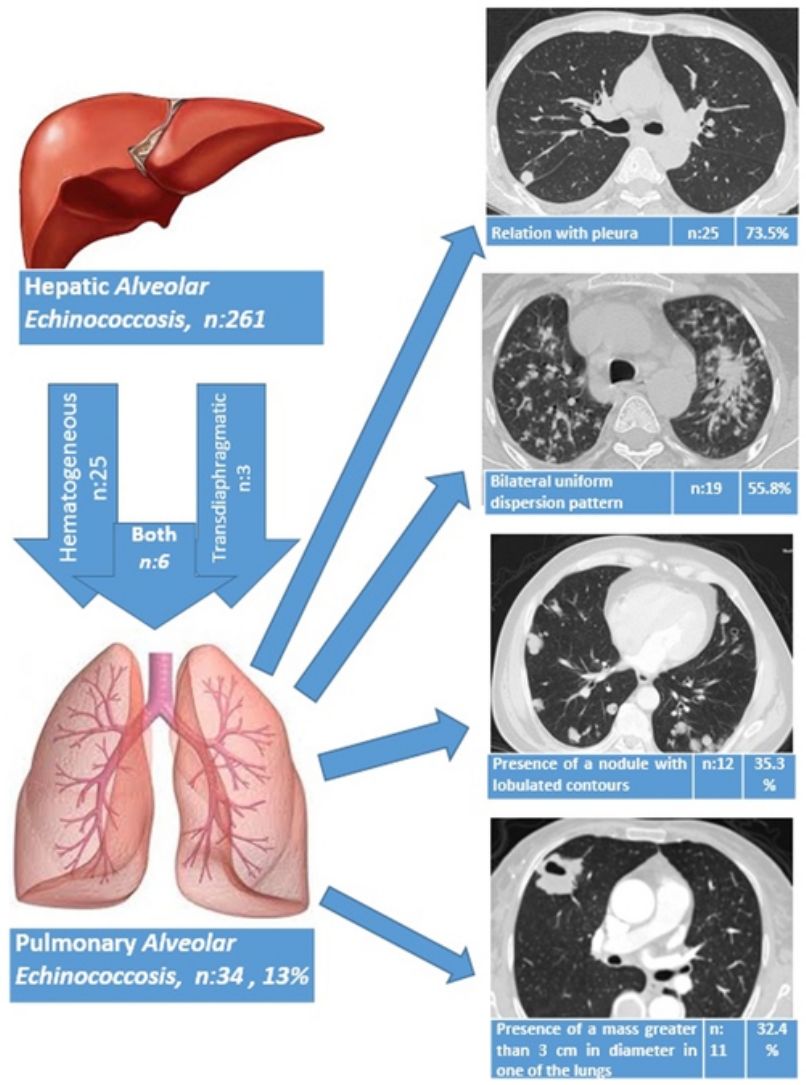
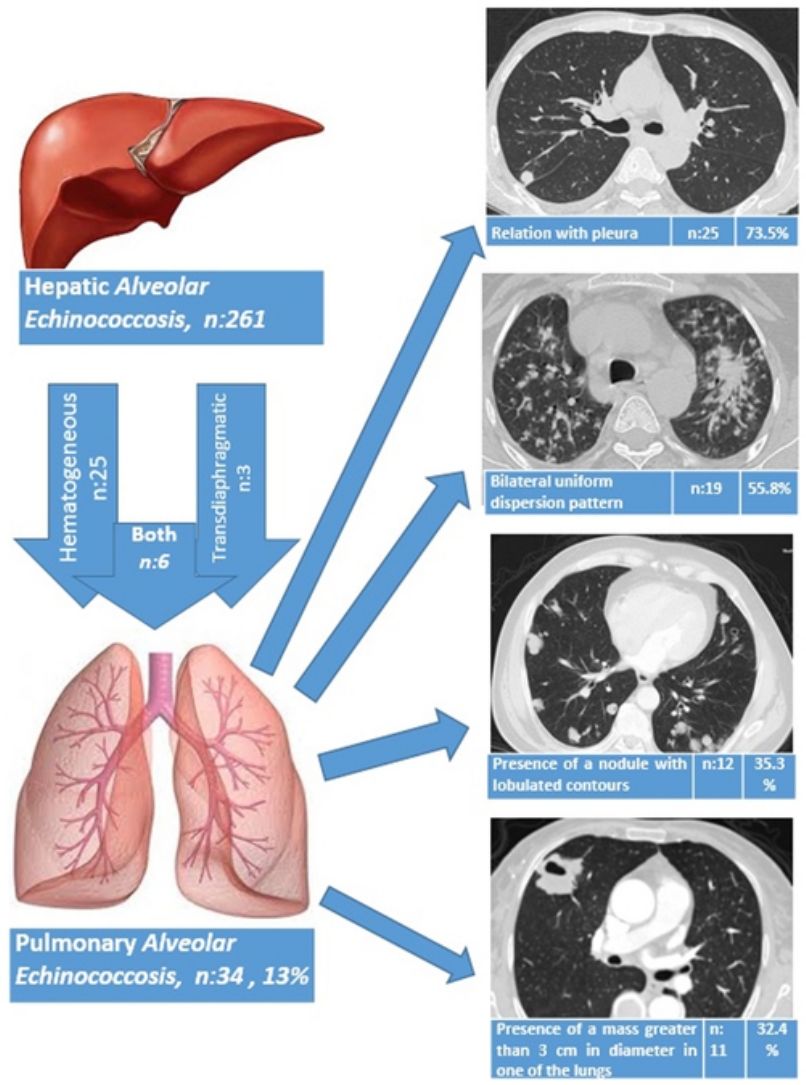
ResultsThe liver was the primary involved organ in all cases. Pulmonary involvement was detected in 13.0% (34/261) of all cases with hepatic alveolar echinococcosis (AE), and three patients (8.8%) had both pulmonary metastasis and brain metastasis. The route of spread to the lungs based on radiological data was hematogeneous in 25 patients (73.5%), transdiaphragmatic in three patients (8.8%) and both hematogeneous and transdiaphragmatic in six patients (17.7%). AE showed bilateral involvement in 19 patients (55.9%), whereas only the right lung was involved in 12 patients (35.3%) and the left lung in three patients (8.8%). Of the patients, five underwent surgery due to PAE and 29 patients received medical therapy with albendazole. A total of three patients died during the follow-up period (2, 5 and 10 years after the diagnosis of PAE), while 31 patients continued with follow-up and treatment for a mean duration of 5.4±3.8 years (1–14 years).
ConclusionsPatients with hepatic AE must, as a matter of course, be screened for possible lung involvement. Albendazole therapy may slow down disease progression in patients with widespread pulmonary involvement who are not eligible for surgery.
La equinococosis alveolar con afectación pulmonar (PAE) es una enfermedad crónica causada por Echinococcus multilocularis, cuya incidencia es muy baja en los países desarrollados.
MétodosEstudio unicéntrico, retrospectivo en el cual se diagnosticaron 34 pacientes con PAE entre enero de 2001 y febrero de 2019 (15 varones y 19 mujeres, edad media: 52,4±15,8 años, rango de edad: 28-78 años) en la Escuela Médica Univesitaria de Ataturk, Erzurum, Turquía.
ResultadosEn el total de los casos incluidos en el estudio el hígado fue el principal órgano afectado. La afectación pulmonar se detectó en el 13% (34/261) de los casos con equinococosis alveolar (AE), y 3 pacientes (8,8%) presentaron tanto metástasis pulmonar como cerebral. De acuerdo con los datos radiológicos, la propagación a los pulmones fue por vía hematógena en 25 pacientes (73,5%), transdiafragmática en 3 pacientes (8,8%) y tanto hematógena como transdiafragmática en 6 pacientes (17,7%). Diecinueve pacientes (55,9%) presentaron PAE con afectación pulmonar bilateral, mientras que 12 pacientes (35,3%) presentaron afectación solo del pulmón derecho y 3 (8,8%) solo del izquierdo. De todos los pacientes, 5 fueron sometidos a cirugía debido a la PAE y 29 recibieron tratamiento médico con albendazol. Tres pacientes fallecieron durante el período de seguimiento (2,5 y 10 años después del diagnóstico de PAE), mientras que 31 continuaron con el seguimiento y el tratamiento durante 5,4±3,8 años de media (1-14 años).
ConclusionesLos pacientes con AE hepática se deben cribar de manera rutinaria para detectar una posible afectación pulmonar. El tratamiento con albendazol puede ralentizar la progresión de la enfermedad en pacientes con afectación pulmonar extendida que no son aptos para cirugía.
Alveolar echinococcosis (AE) is a chronic and progressive disease that primarily involves the liver, and that is caused by the larval form of the taeniid cestode Echinococcus multilocularis (E. multilocularis).1 AE is particularly prevalent in the northern hemisphere and is endemic in Central Europe, North America and Eastern Asia. It is rarely observed in Western Europe.2–7 The annual incidence in Central Europe ranges approximately from 0.02 to 1.4 per 100,000 persons.8 Cases in Turkey have mostly been reported from the Eastern Anatolian Region of the country.1
Foxes, wild dogs, wolves and coyotes act as the definitive host in the life cycle of E. multilocularis, while rodents, deer and bison are intermediate hosts. Domestic dogs and cats can also be infected by and transmit the infection to humans, either directly or by contaminating food with parasite eggs.9 The adult form of the parasite is particularly found in the intestine of such predators as the red fox. Parasite eggs enter the environment through the feces of the animal. DNA indicating the presence of parasite eggs was found to be present in up to 36% of forest fruits, mushrooms and garden vegetables in areas that are endemic for the disease.10 Upon accidental ingestion of these eggs by humans, the metacestode develops into a multivesicular lesion showing a tumor-like growth pattern, particularly in the liver. Humans can become infected through ingestion of food or water contaminated by parasite eggs or by physical contact with domestic or wild animals harboring the parasite in their intestines.
E. multilocularis cysts show a slow growth pattern, with an estimated incubation period of 5–15 years.6,9 In the absence of a limiting parasite- or host-derived membrane, the exogeneous budding and growth of the cyst results in the infiltration of adjacent tissues, causing pressure necrosis of the surrounding host tissues. Microscopically, the cyst is lined by a thin laminated membrane that contains minimal or no germinal layer.9 The liver is the primary site for cyst development in almost all patients. AE behaves like a malignant tumor; the cyst extends to the adjacent structures by invading and destroying tissue and can metastasize to the lungs, brain and other organs.1
Due to the low incidence of AE and the rare occurrence of pulmonary involvement, there are only a few case reports in literature regarding PAE. The present study describes the clinical, laboratory and radiological characteristics of patients with PAE and evaluates the outcomes of treatment methods.
Material and methodsThis single-center, retrospective study included 34 patients who were diagnosed with PAE between January 2001 and February 2019 (15 males, 19 females, mean age: 52.4±15.8 years, age range: 28–78 years) in Ataturk University Medical School, Erzurum, Turkey. The medical history of all patients was obtained and their physical examination findings were recorded. Complete blood count, biochemical parameters and coagulation tests were obtained for all patients. In a radiological workup, posteroanterior chest X-rays and computed tomography (CT) scans were obtained from all patients. An echinococcus IgG ELISA test (NovaLisa, NovaTec Immundiagnostica GMBH, Germany) was performed on 27 patients. Data on age, gender, symptoms, type of pulmonary dissemination, localization, diagnostic methods, radiological findings, involvement of organs other than the lungs, therapies administered and survival were recorded.
A histopathological diagnosis was established in all patients with hepatic lesions as the primary site of involvement. If the lesion in the liver showed continuity with the diaphragm and lung, it was evaluated as a trans-diaphragmatic invasion. Cysts were located near the diaphragm, but those with a clear diaphragm were considered hematogenous metastasis. Pulmonary involvement was confirmed histopathologically by bronchoscopic endobronchial biopsy in 2 patients with endobronchial involvement and by wedge resection for therapeutic purpose in five patients. In the other 27 cases, pulmonary involvement was diagnosed based on clinical and radiological findings by the radiologists and thoracic surgeons from the same team. The 15 patients that were considered to have pulmonary involvement were excluded from the study by the same team by founding suspected. Twenty-seven patients were followed up for years and pulmonary involvement was confirmed.
ResultsThe echinococcus IgG ELISA test was positive in 22 cases and negative in 5 cases. The liver was the primary involvement site in all cases. Pulmonary involvement was detected in 13.0% (34/261) of all cases with hepatic AE. Of the total, three patients (8.8%) had pulmonary metastasis simultaneously with brain metastasis. PAE was diagnosed in 10 patients (29.4%) who had been diagnosed previously with hepatic AE, whereas a diagnosis of PAE was established simultaneously with hepatic AE in 24 patients (70.6%). The mean time for the diagnosis of PAE among patients with previously diagnosed hepatic AE was 3.9 years (1–9 years).
Respiratory symptoms were noted in 21 (61.9%) patients (16 had dyspnea, 13 had chest pain, 12 had cough, and one had hemoptysis), and 13 patients (38.2%) were asymptomatic in terms of the respiratory system, with PAE detected incidentally during a radiological examination.
The route of spread to the lungs based on radiological data was hematogeneous in 25 patients (73.5%), transdiaphragmatic in three patients (8.8%) and both hematogeneous and transdiaphragmatic in six patients (17.7%). AE showed bilateral involvement in 19 patients (55.9%), whereas only the right lung was involved in 12 patients (35.3%) and the left lung in three patients (8.8%).
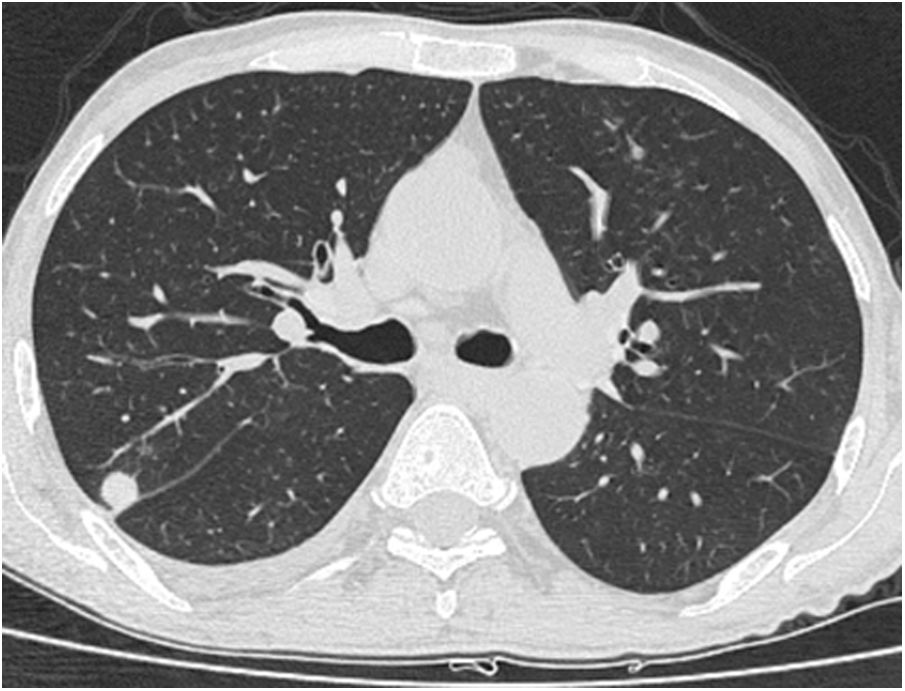
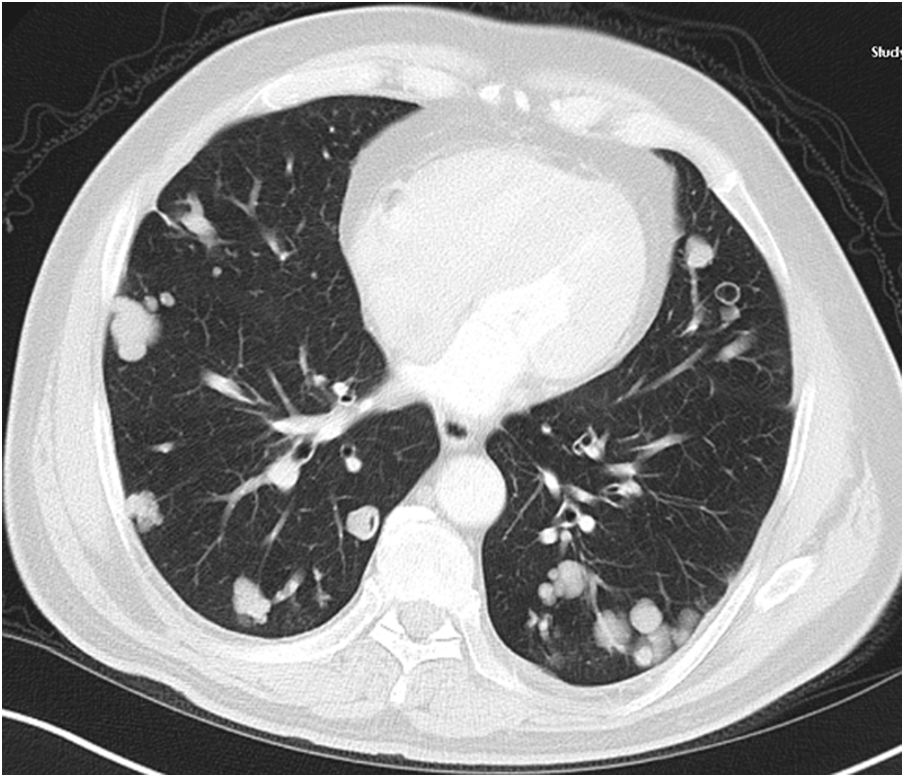
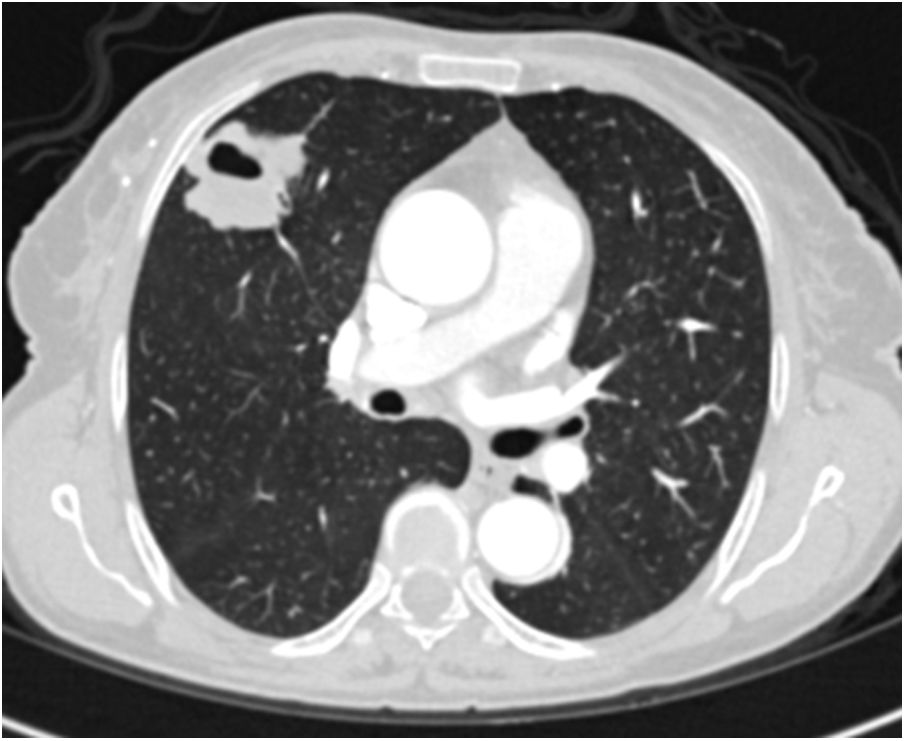
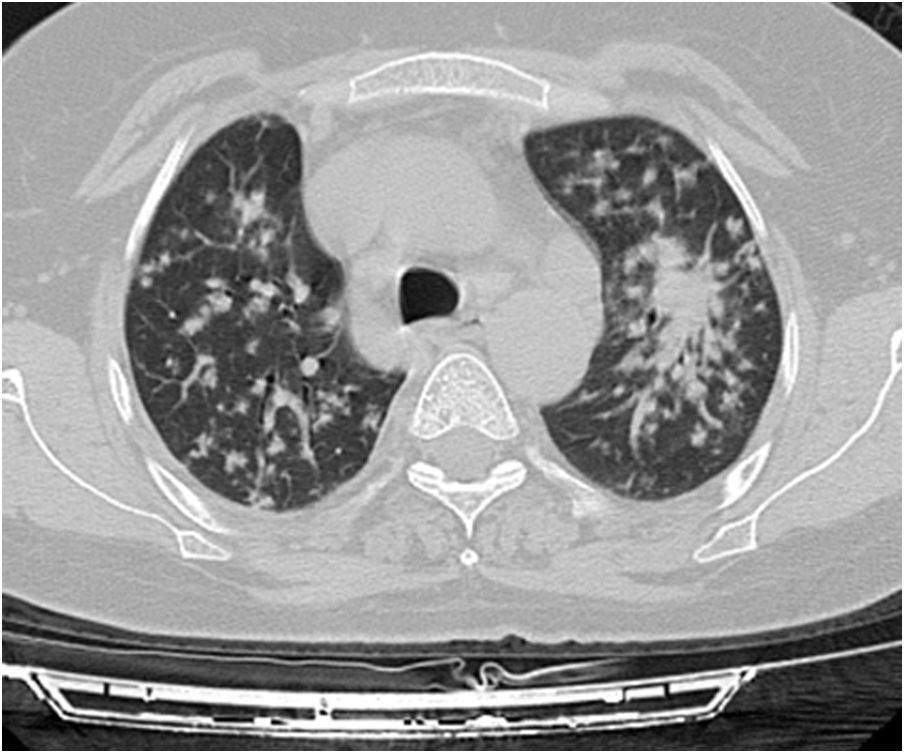
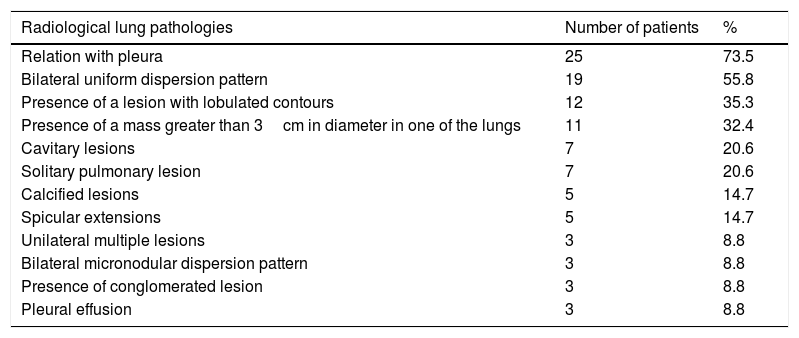
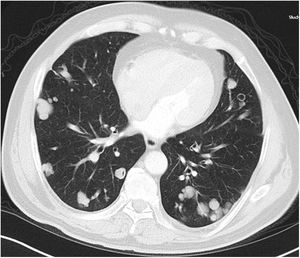
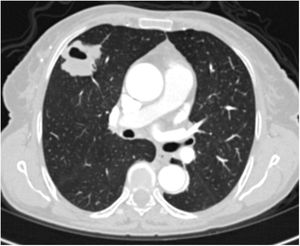
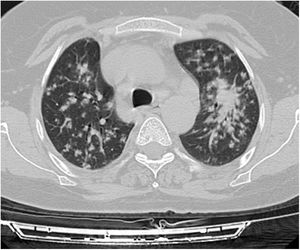
A relationship was identified between the pleura and one or more of the lesions in 25 of the 34 patients diagnosed with PAE (73.5%) (Fig. 1). Following the lesion-pleura relationship, multiple lesions were uniformly scattered throughout the bilateral lung parenchyma in 19 patients (55.8%), seven patients (20.6%) presented with a solitary pulmonary lesion and three patients (8.8%) had multiple unilateral lesions. The diameter of one or more lesions was greater than 3cm in 11 patients (32.4%), only three patients (8.8%) had conglomerated lesions (Fig. 2) and seven patients (20.6%) showed cavitization in some of the lesions. The majority of cavitary mass measured greater than 3cm (Fig. 3). Calcified lesions were recorded in five patients (14.7%), and some of the calcified lesions with lobulated contours had a typical popcorn appearance. Lobulated contours were observed in a significant proportion of non-calcified lesions. Some of the lesions had irregular contours and spicular extensions mimicking malignant processes in five patients (14.7%), while three patients (8.8%) showed multiple micronodular densities with a bilateral uniform dispersion pattern (Fig. 4). The appearance of pulmonary involvement resembled miliary tuberculosis. Parenchymal lesions were accompanied by pleural effusion and parenchymal consolidation in only three patients (8.8%) (Table 1).
Axial thoracic CT scan with lung parenchyma window of a 74-year-old male patient showing multiple lesions in the bilateral lung parenchyma. Some of the lesions show a tendency to cavitization and some show a tendency to conglomeration. Parenchymal lesions tend to have peri-pleural localization in the lung periphery. The patient has been followed for 6 years due to PAE.
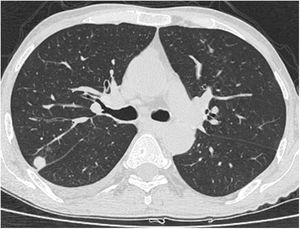
Axial thoracic CT scan with lung parenchyma window of a 61-year-old female patient showing a metastatic mass with a diameter greater than 3cm, mimicking a mass lesion located adjacent to the pleura in the right lung with central cavity and peripheral spicular extensions. The patient has been followed for 4 years due to PAE.
Distribution of presentation among the pulmonary lesions.
| Radiological lung pathologies | Number of patients | % |
|---|---|---|
| Relation with pleura | 25 | 73.5 |
| Bilateral uniform dispersion pattern | 19 | 55.8 |
| Presence of a lesion with lobulated contours | 12 | 35.3 |
| Presence of a mass greater than 3cm in diameter in one of the lungs | 11 | 32.4 |
| Cavitary lesions | 7 | 20.6 |
| Solitary pulmonary lesion | 7 | 20.6 |
| Calcified lesions | 5 | 14.7 |
| Spicular extensions | 5 | 14.7 |
| Unilateral multiple lesions | 3 | 8.8 |
| Bilateral micronodular dispersion pattern | 3 | 8.8 |
| Presence of conglomerated lesion | 3 | 8.8 |
| Pleural effusion | 3 | 8.8 |
Fifteen patients underwent surgical treatment for liver (hepatectomy in 11 cases and liver transplantation in 4 cases) and pulmonary wedge resection in 5 patients. Two patients who have suggested surgical treatment for pulmonary involvement did not accept surgical treatment. Of the 29 patients who did not undergo surgical treatment due to widespread involvements, poor general conditions and non-acceptance of the operation; albendazole treatment (10mg/kg/day) was administered medically every year, 3 months per year. The patients who underwent a pulmonary wedge resection were started on a 3-month course of albendazole therapy (10mg/kg/day) every year for two years to prevent recurrence. No recurrence was observed during the follow-up period (mean 5 years) among the patients that underwent a pulmonary resection. Finally, three patients who were diagnosed with PAE died (2, 5 and 10 years after diagnosis of PAE) due to progression and complications of the hepatic AE, and 31 patients continued follow-up and treatment for a mean duration of 5.4±3.8 years (1–14 years).
DiscussionE. multilocularis causes a severe and mostly fatal infection in humans. Recent years have witnessed an unexpected increase in the prevalence of AE.6 The mean age at diagnosis is 55 years, and while the disease is often observed in later ages, it can also occur in pediatric patients.11 More than 95% of untreated patients die within 10 years of diagnosis.12,13 The mean age in the present study was 52.4±15.8 years, which is consistent with previous studies. Recent clinical studies conducted in our region suggests an increasing incidence of AE.1,13
E. multilocularis does not produce cysts like those observed in E. granulosus infections. E. multilocularis larva develop in the liver and spread by infiltrating adjacent tissues or metastasizing to other organs. Primary extra-hepatic involvement by E. multilocularis occurs rarely.14 Pulmonary metastasis is observed in 7–20% of patients with hepatic involvement.3,5 In a study involving a series of 117 patients with AE, Brasson-Hadni et al.,3 reported pulmonary metastasis in 20% and cerebral metastasis in only 1% of patients. Hepatic involvement always precedes pulmonary involvement. PAE is primarily caused by the hematogeneous spread of hepatic AE lesions,15 but can also occur as a result of the transdiaphragmatic migration of the hepatic AE lesion or the intrathoracic rupture of the cysts into the bronchial tree, pleural space or mediastinum.1,9 Embryophores that cannot attach to the liver migrate through the inferior vena cava or suprahepatic veins to reach the heart and lungs. Embryophores that cannot attach to the lungs return to the heart via the pulmonary veins and then spread throughout the entire body.16E. multilocularis presents as a cancer-like growth in the lungs due to its infiltrative growth patterns and metastatic dissemination. In the present study, the liver was the primarily involved organ in all patients. Pulmonary involvement was observed in 13% of the patients with hepatic AE, and the route of spread in such patients was hematogeneous in 73.5%, transdiaphragmatic in 8.8%, and both hematogeneous and transdiaphragmatic in 17.7%.
Symptoms vary depending on the involved organ and the degree of involvement. AE is detected incidentally in more than one-third of the patients. The initial signs and symptoms are nonspecific: patients may present with weight loss, abdominal pain, fever, jaundice and hepatomegaly, or may suffer from chest pains, shortness of breath, coughing and hemoptysis in the presence of pulmonary involvement.11,17 PAE caused by hematogeneous spread and the intrapulmonary growth of a daughter cyst can remain asymptomatic for approximately 10 years.15 In the present study, 38.2% of the patients had no respiratory symptoms, meaning that further investigation for pulmonary lesions in patients presenting with hepatic lesions in areas with a high prevalence of AE is the key to early diagnosis.
The diagnosis of AE is based primarily on the visualization of parasitic lesions through imaging and serological testing for specific antibodies in the serum. Living in, or having recently visited an endemic area is an important clue in a patient's medical history.18 The clinical presentation and radiological imaging findings of PAE often mimic metastatic malignancies. Diagnosis may be challenging in patients with an atypical presentation.12 A definitive diagnosis is based on a histopathological examination, although serologic tests and radiological imaging studies may be helpful. An Echinococcus IgG-ELISA test was performed on 27 patients in the present study, and 22 patients tested positive, with the diagnosis confirmed by a histopathological examination of the liver samples in all patients in the study. Pulmonary involvement was confirmed by way of a histopathological examination in seven patients, whereas other patients were found to have pulmonary involvement based on clinical and radiological evaluations by the same team of researchers.
A combination of radiological imaging and serologic tests can produce a diagnosis, although serology has the highest sensitivity and specificity. Such E. multilocularis antibodies as Em2 and Em18 are commonly used in the serologic diagnosis of AE, and while it has been reported that these antibodies can discriminate between E. granulosus and E. multilocularis in 95–97% of patients,1 they serve no purpose in monitoring response to therapy as patients show prolonged antibody response after the initiation of treatment. That said, the monitoring of total IgE antibody levels is useful as the levels decrease with successful therapy.
Small nodules in the liver and lung parenchyma are asymptomatic, and the disease may be detected incidentally on ultrasound, CT or MRI performed for other reasons. USG and CT reveal central necrosis, calcifications, irregular contours, and heterogeneous and hypodense foci. MRI is superior in the identification of central necrosis, however, this method does not serve well in the differential diagnosis of calcifications and small lesions. Positron emission tomography-computed tomography (PET-CT) is an important method for detecting the extent of the disease in the body and disease recurrence, and in identifying the viability of the parasite. Imaging studies often show findings that are suspicious for carcinoma or sarcoma. The general medical condition of patients with AE is better than those with suspected malignancy, as was the case in the present study.9 The most common pulmonary findings of CT scans of patients were a relationship with the pleura (73.5%), a bilateral uniform dispersion pattern (55.8%), the presence of a lesion with lobulated contours (35.3%) and the presence of a mass measuring greater than 3cm (32.4%). The lesion showed spicular extensions in 14.7% of the patients, mimicking malignancy.
Treatment should be planned by a multidisciplinary team involving at least one surgeon with extensive experience in clinical parasitology, a radiologist, a hepatologist and an infectious diseases specialist. The World Health Organization (WHO) Informal Working Group on Echinococcosis (IWGE) has described a PNM classification system that is based on imaging findings in an attempt to standardize diagnostic and therapeutic methods for hepatic AE. This classification system draws attention to the malignant course of AE using the criteria of a parasitic mass in the liver (P), the involvement of adjacent organs (N) and metastasis (M).12 The WHO-IWGE guidelines recommend the surgical resection of parasitic foci as the first option for patients with an operable disease, followed by a limited course of chemotherapy.19 The World Health Organization (WHO) recommends that all operable patients undergo chemotherapy for at least 2 years following radical surgery.12 Studies in literature reporting on cases with PAE are rare, and so there is a lack of consensus on the therapeutic approach to patients. The authors of the current manuscript suggest that treatment for PAE should be considered in conjunction with the hepatic lesion. Surgery should be the first option in patients without widespread pulmonary metastases, although most patients with pulmonary involvement are unfortunately not eligible for surgery. Among the patients in the present study, only seven (20.6%) were scheduled for pulmonary resection and two patients rejected surgical therapy. Surgical interventions of the pulmonary lesions were not deemed suitable in the remaining patients (79.4%).
Liver transplantation is necessary in patients with extensive hepatic disease involving the main vascular structures.13 Patients who do not undergo surgery require life-long therapy with benzimidazole derivatives. The 10-year survival rate has increased from 6–25% to 80–83% through the use of albendazole.14,20 The mean expected survival for a 50-year-old patient is 18.2–21.3 years, despite the advances in treatment.7 It is therefore important to prevent the disease by identifying risk factors for the development of disease.
Prolonged benzimidazole (preferably albendazole) therapy is mandatory in all patients that do not qualify for surgery and in patients undergoing radical surgery, the commonly observed side effects of which include alopecia, hepatotoxicity and neutropenia. The patients must be followed up biweekly with tests for impairment in liver function and leukopenia for the first three months of therapy, monthly for one year and then every three months afterwards. Albendazole is metabolized to albendazole sulfoxide in the liver. The blood levels of this active metabolite must be monitored in all patients, particularly those with hepatocellular dysfunction and/or cholestasis. Albendazole is a parasitostatic agent, and rarely eliminates E. multilocularis, and so patients who are not considered suitable for surgery therefore require lifelong therapy to inhibit or at least suppress the growth of the parasite (10–15mg/kg/day, peroral in two divided doses). Despite the limited efficacy, albendazole therapy increases life expectancy to nearly normal levels in patients with AE.12 In the present study, patients who underwent a pulmonary wedge resection were started on 3-month courses of albendazole therapy (10mg/kg/day) every year for two years to prevent recurrence. Furthermore, 29 patients who were deemed unsuitable for surgery received 3-month courses of albendazole therapy (10mg/kg/day) every year and were followed up at regular intervals.
Patients undergoing radical surgery had better outcomes, whereas elderly patients had a poorer prognosis than the younger patients. Torgerson et al.,7 found that gender and the presence of metastasis are not independent prognostic factors for AE-related mortality.
Disease prevention is considerably difficult, as the disease develops through the transmission of parasite eggs to human hosts. The spread of disease can be prevented through the treatment of foxes and wild dogs, although such approaches are expensive, and it has been ascertained that such approaches do not make the desired economic contribution. Studies in literature show that the treatment of animals that cause the spread of the disease as a means of preventing the disease has not produced the desired effect.21 Sero-epidemiological studies of humans, however, may have an economic contribution.
ConclusionIn conclusion, PAE is a tumor-like parasitic disease relatively prevalent in some endemic regions that occurs typically after hepatic involvement. All patients diagnosed with hepatic AE (or with hydatic cyst) must be evaluated for possible pulmonary metastases. The treatment must be decided upon after evaluating PAE together with the hepatic lesion. Surgical therapy must be the first option if the pulmonary metastases are resectable and the hepatic lesion is suitable for resection or liver transplantation. Albendazole therapy may slow disease progression when administered for two years to patients who have undergone surgery, and when administered lifelong to patients with widespread pulmonary involvement who are not eligible for surgery.
Conflict of interestsThe authors declare no conflict of interest.