Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which emerged in Wuhan (China) in December 2019.1 Although most cases are mild, nearly 20% of patients require hospitalization.2 Despite supportive care, one third of hospitalized patients meet the Berlin criteria for acute respiratory distress syndrome (ARDS),3,4 and may need admission to intensive care unit (ICU). Overall mortality ranged 21–26% with advanced age, male sex and comorbidities being the strongest predictors of in-hospital mortality.5,6
Apart from dexamethasone,7 to date the mainstay of treatment if COVID-19 are supplemental oxygen therapy and best supportive care, including invasive mechanical ventilation (IMV) in severe ARDS.8 Prone position (PP) is the only technique that has demonstrated to increase survival in patients with severe ARDS receiving IMV.9 However, due to a sudden increase in hospitalizations, exceeding ICU capacity, PP has been attempted in non-intubated awake patients with COVID-19 and severe ARDS in conventional medical wards,10–12 despite inconsistent results on the impact of this intervention on mortality.13 In this study, we evaluated the impact on in-hospital mortality of PP in spontaneously breathing patients with COVID-19 and severe ARDS.
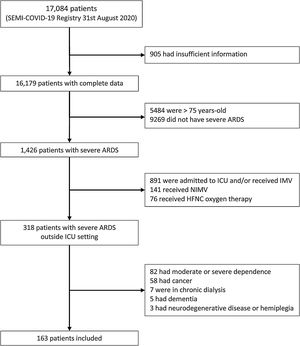
This study is based on the data from the SEMI-COVID-19 Registry. This Registry is an ongoing nationwide, multicenter, observational retrospective cohort of adult patients admitted to Spanish hospitals from March 1st, 2020 because of microbiologically confirmed COVID-19. Characteristics of the Registry are detailed elsewhere.6 In this study we included patients aged 18–75 years old who presented pneumonia and severe ARDS (PaO2/FiO2≤100mmHg)4 during hospitalization. Exclusion criteria were: (a) ICU admission, (b) use of non-invasive mechanical ventilation (NIMV), (c) use of high-flow nasal cannula (HFNC) oxygen therapy, (d) moderate or severe dependence for activities of daily living (e) solid or hematologic cancer, (f) chronic dialysis, (g) neurodegenerative disease or hemiplegia. The primary outcome was death during hospitalization. Patients in whom PP during spontaneous breathing was used for at least one day were compared to those who did not receive this treatment. PP indication and duration were decided at clinical discretion and this information was not recorded in the registry.
Baseline characteristics between groups were compared using Mann–Whitney U test and Fisher's exact test. Univariate logistic regression was used to estimate the crude effect of prone position on mortality, as well as the effect of those baseline variables that were different between both groups (p value<0.10) or considered as clinically relevant. Afterwards, a multivariate logistic regression model was elaborated, including as confounders the variables that showed a univariate effect on mortality or those a priori (outcome-blinded) considered as relevant according to literature review5 and clinical plausibility (age, sex, Charlson Comorbidity Index (CCI), heart failure, altered mental status, and treatment with tocilizumab or corticosteroids). Beginning with the maximum model containing all the variables, we use an investigator-guided backward elimination method to estimate the final model, iteratively excluding non-significant variables until all variables of the model showed p-values<0.05. The goodness of fit of the model was evaluated with the area under the receiver operating characteristics curve (AUC). The 95% confidence interval (95% CI) of AUC was calculated using the binomial exact method. All statistical analyses were performed using Stata 15.2.
Until August 31st, 2020, the SEMI-COVID-19 Registry included 17,084 patients, of which 163 presented severe ARDS and met inclusion criteria and did not meet any exclusion criteria (Fig. 1). Prone position was used in 60 patients (36.8%). Baseline characteristics, treatment received and outcomes in both groups are detailed in Table 1. Patients who receive PP were younger, had less comorbidity, presented higher C-reactive protein and D-dimer levels at admission, and received more corticosteroids and tocilizumab. Besides, although the proportion of patients with altered mental status at presentation did not meet our pre-specified criteria of p-value<0.10 to be considered different between the two groups, we considered the difference clinically relevant (7.8% vs 15.3%). Ninety out of 163 patients (55.2%) died. Patients who died were older and have more comorbidity (Supplementary Table 1). Patients treated with PP presented lower mortality (62.1% vs 43.3%, p=0.0229), with an estimated crude OR of 0.47 (95% CI 0.24–0.89). In the adjusted model, use of PP showed a protective effect on mortality (OR 0.42, 95% CI 0.18–0.98), after adjusting for age, CCI and altered mental status at presentation (Table 2). The AUC of this adjusted model was 0.861 (95% CI 0.799–0.912). Although it could be arguable to remove corticosteroids and tocilizumab as confounders in the adjusted analysis, the exclusion of these variables did not modify the protective effect of PP on mortality, and the accuracy of our adjusted model to predict in-hospital death was very good.
Characteristics of patients according to prone positioning.
| Prone position | p value | Missing values | ||
|---|---|---|---|---|
| No(n=103) | Yes(n=60) | |||
| Demographics and comorbidity | ||||
| Age (years) | 70.81 [60.6–74.2] | 66.57 [59.2–72.4] | 0.0566 | 0 |
| Male sex | 71 (68.9%) | 43 (71.7%) | 0.8596 | 0 |
| Ethnicity | 0.1956 | 6 | ||
| Caucasian | 93 (90.3%) | 45 (75.0%) | ||
| Latin | 8 (7.8%) | 9 (15.0%) | ||
| Other | 1 (1.0%) | 1 (1.7%) | ||
| Smoker status | 0.1900 | 5 | ||
| Never | 60 (58.3%) | 29 (48.3%) | ||
| Former smoker | 31 (30.1%) | 26 (43.3%) | ||
| Current smoker | 9 (8.7%) | 3 (5%) | ||
| Obesity | 29 (29.3%) | 19 (33.3%) | 0.5950 | 7 |
| Charlson Index | 1 [0–3] | 0 [0–0] | 0.0182 | 1 |
| Hypertension | 64 (62.1%) | 35 (58.3%) | 0.7396 | 0 |
| Diabetes mellitus | 38 (36.9%) | 19 (31.7%) | 0.6097 | 0 |
| COPD | 16 (15.5%) | 5 (8.3%) | 0.2302 | 0 |
| Obstructive sleep apnea | 9 (8.8%) | 10 (16.7%) | 0.2048 | 1 |
| Heart failure | 16 (15.5%) | 2 (3.3%) | 0.0187 | 0 |
| Clinical presentation | ||||
| Duration of symptoms (days) | 7 [4–9] | 7 [5–10] | 0.2264 | 0 |
| Respiratory rate>20breaths/min | 49 (48.5%) | 28 (47.5%) | 1.0000 | 3 |
| Altered mental status | 8 (7.8%) | 9 (15.3%) | 0.1835 | 2 |
| C-reactive protein (mg/L) | 95.2 [35–171.3] | 155 [85.6–226] | 0.0092 | 6 |
| D-dimer (ng/mL) | 834.5 [356–1574] | 1030 [563–1650] | 0.1700 | 28 |
| Lymphocyte count (cells/μL) | 800 [560–1130] | 800 [610–1275] | 0.6720 | 1 |
| SaO2/FiO2at admission | 400 [241.7–438.1] | 409.5 [306.3–438.1] | 0.6075 | 10 |
| Treatments received | ||||
| Lopinavir/ritonavir | 71 (68.9%) | 33 (55.0%) | 0.0914 | 0 |
| Hydroxychloroquine | 89 (86.4%) | 56 (93.3%) | 0.2044 | 0 |
| Remdesivir | 2 (2.0%) | 0 (0.0%) | 0.5307 | 1 |
| Corticosteroids | 62 (60.2%) | 52 (86.7%) | 0.0004 | 0 |
| Tocilizumab | 21 (20.4%) | 25 (41.7%) | 0.0064 | 0 |
| Antibiotic use | 96 (94.1%) | 54 (98.2%) | 0.4227 | 6 |
| Beta-lactamic | 79 (77.5%) | 40 (74.1%) | 0.6939 | 7 |
| Quinolone | 21 (20.4%) | 6 (10.0%) | 0.1270 | 8 |
| Azithromycin | 54 (52.4%) | 40 (66.7%) | 0.0175 | 6 |
| Outcomes | ||||
| In-hospital mortality | 64 (62.1%) | 26 (43.3%) | 0.0229 | 0 |
| Length of stay (days) | 9 [4–16] | 13 [7.5–21] | 0.0031 | 0 |
Continuous variables are expressed as median and interquartile range [Q1–Q3]. Categorical variables are expressed as number and percentage.
COPD: chronic obstructive pulmonary disease.
Effect of prone position and potential confounder variables on in-hospital mortality.
| Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Prone position | 0.47 (0.24–0.89) | 0.42 (0.18–0.98) |
| Age (years) | 1.12 (1.07–1.17) | 1.11 (1.05–1.16) |
| Male sex | 0.89 (0.46–1.76) | |
| Charlson Index | 2.28 (1.65–3.17) | 1.97 (1.44–2.70) |
| Heart failure | 4.67 (1.30–16.81) | |
| Altered mental status | 4.29 (1.18–15.58) | 8.20 (1.75–38.43) |
| C-reactive protein (mg/L) | 1 (0.99–1.00) | |
| D-dimer (ng/mL) | 1 (1.00–1.00) | |
| Lopinavir/ritonavir | 0.86 (0.45–1.63) | |
| Corticosteroids | 0.49 (0.24–0.98) | |
| Tocilizumab | 0.27 (0.13–0.56) |
Maximum model included age, male sex, Charlson Index, heart failure, altered mental status, treatment with corticosteroids and treatment with tocilizumab.
Despite PP is a well-established evidence-based practice in patients with typical ARDS undergoing IMV, there is very limited evidence of its use in non-ventilated awake patients and derived mostly from case reports and uncontrolled cohorts using a wide variety of PP protocols.13,14 Besides, in most reports the use of respiratory support is inconsistent, with patients receiving NIMV or HFNC oxygen therapy, thus making very difficult to know the true effect of PP.13 Despite most studies reported an improvement in oxygenation while patients were in PP and even after PP,10 its impact on hard outcomes such as intubation or death remains unclear. In fact, it has been pointed out that improvement in oxygenation during PP may result in delayed intubation and IMV with eventually poor outcomes.15 However, in a recent retrospective cohort with 166 patients, Padrão et al. studied the impact of PP in patients who required supplemental oxygen (but did not receive NIMV or HFNC oxygen) and found no differences on the need of intubation at 15 days.16 A possible explanation could be that the benefits of PP are related to a reduction in ventilator-induced lung injury rather than with better oxygenation.17
In our study, we excluded older patients because they could have greater comorbidities and most of them would not benefit from ICU admission in a context of pandemic crisis. This scenario happened in the first weeks of COVID-19 pandemic in Spain and other developed countries and is still ongoing in resource-limited settings.
The main strength of our study is the use of a hard endpoint as mortality as the primary outcome, and the large number of patients included in comparison to previous studies on this topic. Besides, contrary to previous reports, our cohort is homogeneous regarding respiratory support, as we only include patients with spontaneous breathing receiving oxygen supply through a conventional oxygen mask (either Venturi masks or rebreathing masks).
This study also has several limitations. The main one is the lack of detailed information on indication, timing, or duration of PP, and as a result, it is probable that PP was heterogeneous across the cohort. Secondly, we do not have information on the reasons why our patients with severe ARDS were not admitted to the ICU, and given the multicenter nature of the cohort, these reasons could be different between hospitals, potentially resulting in a selection bias. To minimize the risk of selection bias we decided to analyze only COVID-19 patients ≤75 years and severe ARDS, as we believe that the main reason these patients remained in conventional wards was the unavailability of ICU resources.
In summary, the use of prone position in non-intubated COVID-19 patients ≤75 years with severe ARDS and without additional respiratory support, may be associated with improved survival in situations where ICU beds are not available, suggesting that it could be useful in pandemic crisis scenarios. However, randomized controlled trials are needed to confirm our results and to establish adequate protocols of PP in non-intubated patients.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that there is no conflict of interest to report.
We want to thank Martín Fabregate Fuente for providing statistical support. We also want to gratefully acknowledge all the investigators who participate in the SEMI-COVID-19 Registry. We also thank the SEMI-COVID-19 Registry Coordinating Center, S&H Medical Science Service, for their quality control data, logistic and administrative support.
A complete list of the SEMI-COVID-19 Network members is provided in the Appendix (Supplementary Material).