Necrotizing pneumonia is a serious form of lung parenchymal disease associated with abscesses and cavitation. In children, the main etiology of pneumonia remains pneumococcal infection, although the incidence is falling thanks to the use of the pneumococcal vaccine, whereas infections with Staphylococcus spp. are increasing slightly. The main complications are parapneumonic effusion, respiratory distress, pneumothorax, and bronchopleural fistula.1–3
Persistent air leaks (PAL) are those that do not resolve despite placement of a chest drain for more than 7 days. Endobronchial valves (EBV) that allow air and accumulated secretions to be expelled while preventing air from being inhaled are a minimally invasive solution for these fistulas in adults, but this technique is exceptional in children under 5 years of age.4 This paper describes the use of an EBV to treat persistent air leakage due to necrotizing pneumonia in a 4-year-old child.
Our patient was originally from Peru and has been living in Spain since 2019; his only clinical history of interest is an autism spectrum disorder. He was hospitalized for bilateral pneumococcal pneumonia with complicated right unilateral parapneumonic effusion, for which antibiotic treatment with clindamycin and cefotaxime was started and a chest tube was placed. Shortly thereafter, he developed hypoxemic respiratory failure and was admitted to the ICU. Five days after ICU admission, the patient presented with right tension pneumothorax, which led to a 6-min cardiorespiratory arrest requiring orotracheal intubation. As a result of an acute respiratory distress syndrome and refractory hypoxemia, the patient required extracorporeal membrane oxygenation (ECMO) for 17 days.
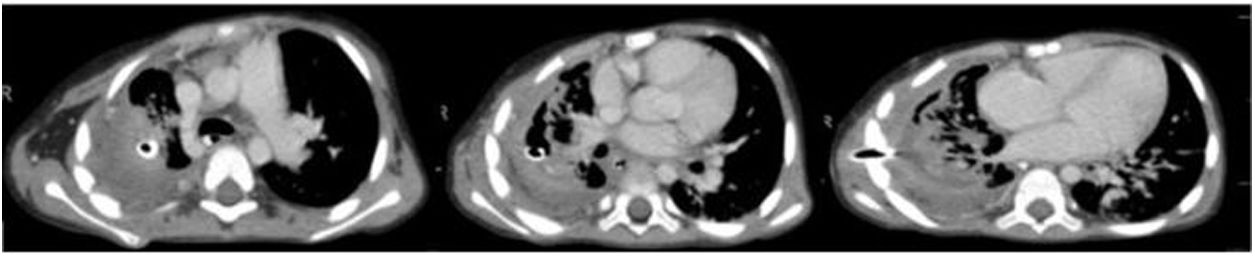
A chest CT scan was performed 24 days after admission to the ICU (Fig. 1) that revealed necrotizing pneumonia with signs of a probable bronchopleural fistula. The patient improved progressively, and a tracheostomy could be performed 1 month after admission. Invasive ventilation was disconnected on day 45, but the air leak through the chest tube persisted.
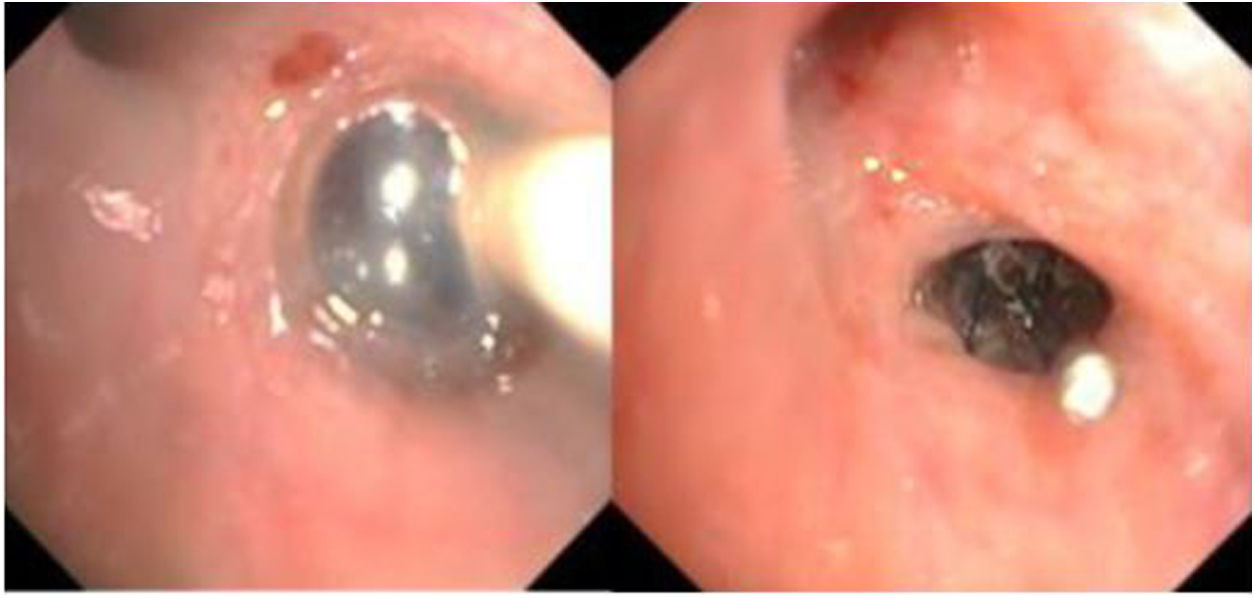
The case was discussed with the Respiratory Endoscopy Unit, and the possibility of placing an EBV in the right upper lobe to treat the PAL was proposed. Valve placement was performed 1 week later in the operating room under sedation and analgesia managed by the anesthesiologist. A BF-Q180 video bronchoscopy (Olympus-Europe, Hamburg, Germany) was introduced through the mouth while the patient was ventilated via the tracheostomy by jet ventilation (high pressure ventilation at a supraphysical frequency followed by active expiration, indicated especially in airway and thoracic procedures). A bronchial occlusion balloon was placed in the tertiary bronchi to check whether the air leak was located in the right upper lobe. This confirmed that the source of the leak was in the posterior segmental bronchus (Fig. 2a). The smallest Spiration® EVB (5mm) was placed (Fig. 2b), and the air leak ceased almost immediately. The patient's subsequent course was satisfactory, the drainage tube could be removed after 7 days, and he was discharged from hospital 15 days after the procedure. The EBV was removed 4 weeks later with no complications or recurrence of the fistula.
The ACCP consensus5 for spontaneous pneumothorax recommends surgical management of patients with pneumothorax due to PAL, and conservative treatment is preferred in patients in whom surgery is contraindicated and in some recurrent cases. Conservative therapeutic techniques include continuous aspiration through a chest tube, chemical pleurodesis, or endobronchial techniques including EBV, of which the most commonly used are Spiration® IBV (Olympus, Tokyo, Japan) and Zephyr® (Pulmonx Corp, Redwood City, CA, USA).
EBVs were first designed for endoscopic volume reduction treatment in severe emphysema following the publication of the NETT study,6 but they have also been used in recent years for the treatment of PAL.
Reed et al.7 reported that 2 of 3 EBVs placed in patients with PAL due to parenchymal infection were effective: the third failed to achieve complete bronchial occlusion and was thus ineffective. The study by Cordovilla et al.8 also shows that EBV placement is an effective treatment for PAL due to secondary pneumothorax: the authors found it to be a safe procedure that resolved 75% of cases. Fiorelli et al. conducted a multicenter study9 in which 88% of PALs resolved with EBV placement, and of these, 68% resolved within the first 24h of placement with no significant differences among the different etiologies. Chest tubes were removed between 8 and 16 days, and valves within 6-8 weeks. These two studies demonstrate the efficacy of EBV in adults, since the median ages were 64.0 and 68.5 years, respectively.
In a systematic review10 published in 2017, PAL was associated with higher morbidity and mortality, and longer hospital stay, and occurred most frequently in the upper lobes. Of all the cases included in the analysis, only 4 were children – all over 5 years of age – and 21% of the PALs were due to lung infection. EBV results are good with a low complication rate, allowing withdrawal in 40% of cases. The authors therefore conclude that EBVs are a useful treatment, particularly in high-risk patients.
As we have seen, very few groups have used EBV instead of other more conventional procedures in the treatment of EBV in children. Two4,11 groups implanted EBV in 7 children with good results and good tolerance in all cases. In all published cases, ages ranges from 16 months to 18 years. However, only 2 of the patients were children under 5 years of age.
Our patient is of particular interest because of his age and presentation, consisting of an air leak due to necrotizing pneumonia that led to a prolonged stay in the ICU. The air leak resolved after EBV placement, so the patient could be discharged and the device was removed 4 weeks later without complications
FundingThis paper has not received any funding.