N-acetylcysteine (NAC) is a mucolytic agent with antioxidant properties. Oxidative stress is a key pathogenic mechanism in chronic respiratory conditions such as COPD and chronic bronchitis (CB). In these meta-analyses we investigated the efficacy of NAC in subjects with COPD or CB, the latter being a potential pre-COPD condition (CB/pre-COPD).
MethodsThe meta-analyses were conducted according to PRISMA guidelines. Exacerbations were assessed using total number of exacerbations. Improvement in patients’ respiratory symptoms and/or patients quality of life (QoL) were measured by validated tools or assessed at the end of the study.
ResultsTwenty studies were included, of which seven evaluated NAC in patients with symptoms of CB/pre-COPD as entry criterion.
NAC treated patients showed a significant reduction of the incidence of exacerbations as compared to placebo both in COPD (IRR=0.76; 95% confidence interval (CI) 0.59–0.99) and CB/pre-COPD (IRR=0.81; 95% CI 0.69–0.95). Sensitivity analyses in studies with duration higher than 5 months, confirmed the overall results.
CB/pre-COPD patients treated with NAC were significantly more likely to experience an improvement in symptoms and/or QoL compared to placebo (odds ratio (OR)=3.47; 95% CI 1.92–6.26). A similar trend was observed in the few COPD studies evaluable.
Sensitivity analyses showed a significant association of NAC with improvement in symptoms and/or QoL both in CB/pre-COPD and COPD patients.
ConclusionsThese findings provide novel data of NAC on the improvement in symptoms and QoL in addition to prevention of exacerbations in COPD and CB/pre-COPD.
PROSPERO registry no. CRD42023468154.
Chronic obstructive pulmonary disease (COPD) is a chronic lung condition characterized by airflow limitation affecting millions of people and representing the third leading cause of death worldwide.1,2
A new personalized medicine strategy based on the identification of “treatable traits” has been recently proposed for the management of patients with chronic pulmonary diseases.3 This approach allows the identification of core treatable traits associated with outcomes for which targeted treatment is available. Chronic bronchitis (CB) is one of the most prevalent pulmonary traits in patients with COPD, together with airflow limitation, breathlessness, chronic sputum production and frequent chest infections.4–7 Chronic respiratory symptoms are associated with respiratory hospitalizations and death even in individuals with normal spirometry.8 The term pre-COPD has been proposed to identify subjects with no airflow limitation (post bronchodilator FEV1/FVC ≥0.7) but exposed to risk factors and presenting with imaging alterations and/or respiratory symptoms (including chronic cough)/and or lung function abnormalities.8,9 They experience significant respiratory symptom burden and are at greater risk of developing COPD.8 Identifying pre-COPD patients before airflow obstruction can be detectable may enable modification of the course of disease and prevent the development of irreversible airflow limitation.8 Patients with CB but no airways obstruction on spirometry belong to the pre-COPD category.
Oxidative stress is a key pathogenic mechanism in COPD which is primarily caused by exposure to cigarette smoke or other environmental pollutants. In the lungs, this can result in inflammation, structural changes, and decreased lung function. Oxidative stress can also impair the immune system's ability to fight infections, contributing to making COPD patients more susceptible to respiratory infections.2 N-acetylcysteine (NAC) is an antioxidant with multiple activities: it acts as a reactive oxygen species scavenger and as a precursor of reduced glutathione, and it has mucolytic properties. Treatment with NAC has been primarily evaluated on exacerbation's prevention, whereas its efficacy in reliving COPD symptoms has been barely investigated and never considered in meta-analysis studies.10–12 In addition, the evaluation of the efficacy of NAC in different patients (e.g. with or without stablished airflow obstruction) is still lacking, and no information is available on tailoring NAC therapy in these populations. This missing clinical information is substantial for a more personalized approach to therapy.3
We performed a systematic review and meta-analysis of the effects of NAC in the populations of (a) symptomatic patients with CB and no diagnosis of COPD and (b) COPD patients. The outcomes of interest were exacerbations, respiratory symptoms, and quality of life.
Materials and MethodsThis review was conducted according to Preferred Reporting Items of Systematic review and Meta-Analysis (PRISMA) guidelines.13
Search Strategy and Selection ProcessWe performed a systematic literature search on the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed and ClinicalTrials.gov from their inception up to February 05, 2024. No language restrictions were applied. In addition, we examined citations of relevant previous published meta-analyses to identify other pertinent studies.
In this registered systematic review (PROSPERO registry no. CRD42023468154), all randomized clinical trials (RCT) which evaluated adult patients with COPD or CB receiving oral N-acetylcysteine versus placebo for at least two consecutive months have been included. The search terms “Chronic obstructive pulmonary disease”, “COPD” and “Chronic Bronchitis” were used to identify the disease and “N-acetylcysteine”, “acetylcysteine” and “NAC” for the treatment. Details about the search terms used in each database are provided in the Appendix Table 1.
COPD inclusion criteria were defined using pulmonary function tests as per the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.2 Chronic bronchitis inclusion criteria were defined using the British Medical Research Council and GOLD14 definition (cough and sputum on most days during at least three consecutive months for at least two successive years). All doses of NAC were included.
Data ExtractionFrom each selected study, we extracted information about year of publication, study duration, NAC doses, number of patients who completed study treatment, disease characteristics, age, sex, smoking habits, smoking history, definition of exacerbation, COPD and CB diagnosis, respiratory symptoms, spirometry measures, radiographic abnormalities and inhaled therapies.
OutcomesThe outcomes assessed in the analyses were: (a) exacerbations of the underlying chronic condition and (b) improvement of patients’ respiratory symptoms and quality of life (QoL).
Exacerbations were assessed using total number of exacerbations in patients who completed the study and number of patients with no exacerbations during the study period.
Improvement in patients’ respiratory symptoms and/or patients quality of life (QoL), measured as the number of patients who experienced an improvement in respiratory symptoms or quality of life by either using validated tools, or using the patients’ and physicians’ assessment at the end of the study period.
For all outcome measures, the total number of patients who completed the study period was considered.
Data AnalysisWe used odds ratios (ORs) for dichotomous data and incidence rate ratios (IRRs) for count data and reported results with 95% confidence intervals (CIs). Incidence rate ratios were measured by dividing the number of events by total number of person-months assuming constant underlying risk.
A random-effects model was used to pool effect sizes and account for anticipated between-study heterogeneity.15 Knapp–Hartung adjustments16 were used to calculate the confidence interval around the pooled effect.
The Paule–Mandel procedure17 was used to estimate the between-study variance.18–20 Between-study variance confidence intervals have been estimated following the recommendations of Veroniki and using the Q-Profile method.20
For binary outcomes, the Peto method was used to calculate the weights of the studies. Natural logarithms of the incidence rate ratios have been combined across studies using the generic inverse-variance method,21,22 as suggested in Higgins et al.23
All analyses were performed distinctly on two subgroups of studies defined a priori:
- 1.
Studies with inclusion criterion of chronic bronchitis and no diagnosis of COPD: they include CB and pre-COPD subjects: CB/pre-COPD group.
- 2.
Studies with inclusion criterion of COPD using PFT as defined by international criteria (GOLD): COPD group.
Sensitivity analyses were planned by considering studies that were excluded from the original analysis due to uncertainty in the diagnostic of the population. Studies that reported spirometry data indicating a mixed population of patients with COPD and patients with chronic bronchitis/pre-COPD without any way of distinguishing them.
Additional sensitivity analyses were performed distinguishing studies with duration less or equal to 5 months and studies with duration higher than 5 months for the following endpoints: total number of exacerbations in patients who completed the study and number of patients without exacerbations during the study period.
In the CB/pre-COPD subgroup, the analysis by duration was not performed because the 6 studies that were included in the original analysis have a duration higher than 5 months.
The primary outcome of interest was improvement in items related to symptoms for the CB/pre-COPD subgroup and exacerbations for the COPD subgroup.
Other sensitivity analyses were performed by dose level. Each dose level was considered separately except for 1200mg and 1800mg which were combined. This analysis was performed on the following endpoints: total number of exacerbations in patients who completed the study and number of patients without exacerbations during the study period.
The sensitivity analysis by dose was not performed on the improvement of patients’ respiratory symptoms and quality of life (QoL) endpoint, because for the COPD subgroup, all studies have the same dose (600mg) and for the CB/pre-COPD subgroup, each of the three papers has a different dose level.
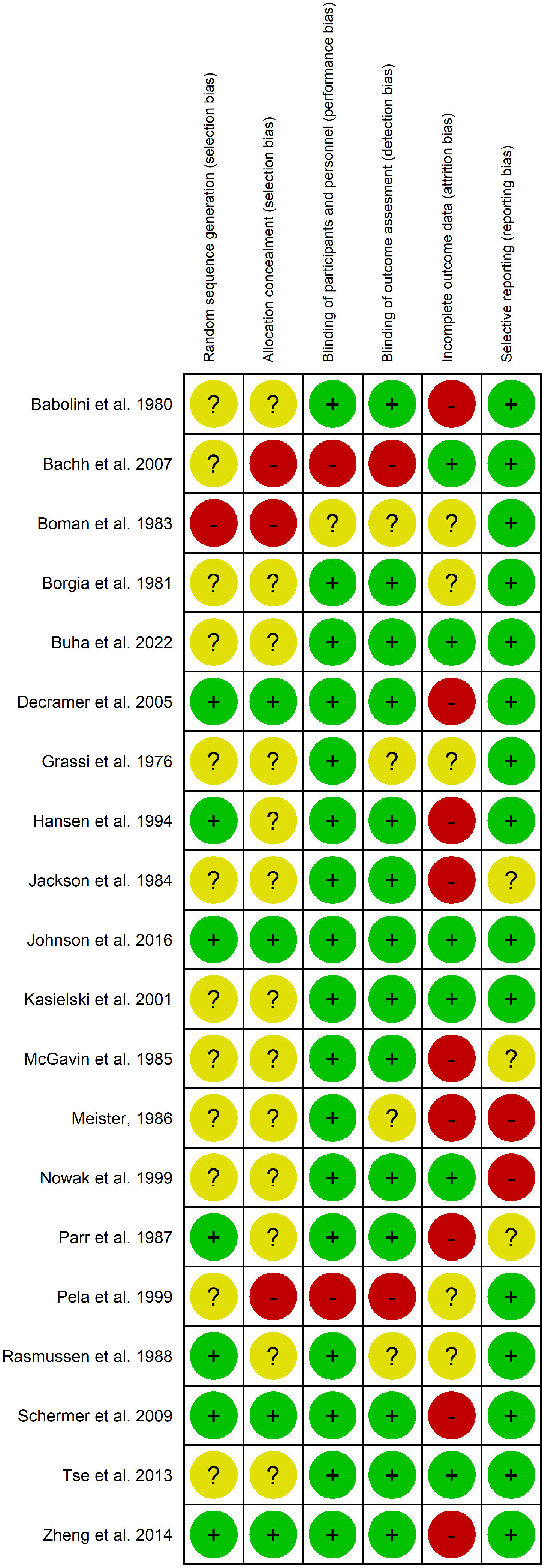
Assessment of Bias and HeterogeneityWe assessed the risk of bias in included studies in selection, allocation, performance, detection, attrition, or reporting according to the Cochrane Collaboration's tool trials.24 Each study was judged for each of the different tool dimensions either at low or high risk of bias (Appendix Table 2). If insufficient information was available, we reported an unclear risk of bias (Fig. 5).
Contour enhanced funnel plots (with confidence regions at p=0.05, p=0.025 and p=0.01) were used to explore possible small-study and publication biases if enough studies were available (i.e., more than 10).
Between-study heterogeneity was assessed by means of Chi-squared test, I2 statistic25,26 and prediction intervals (PIs),25,26 when applicable.
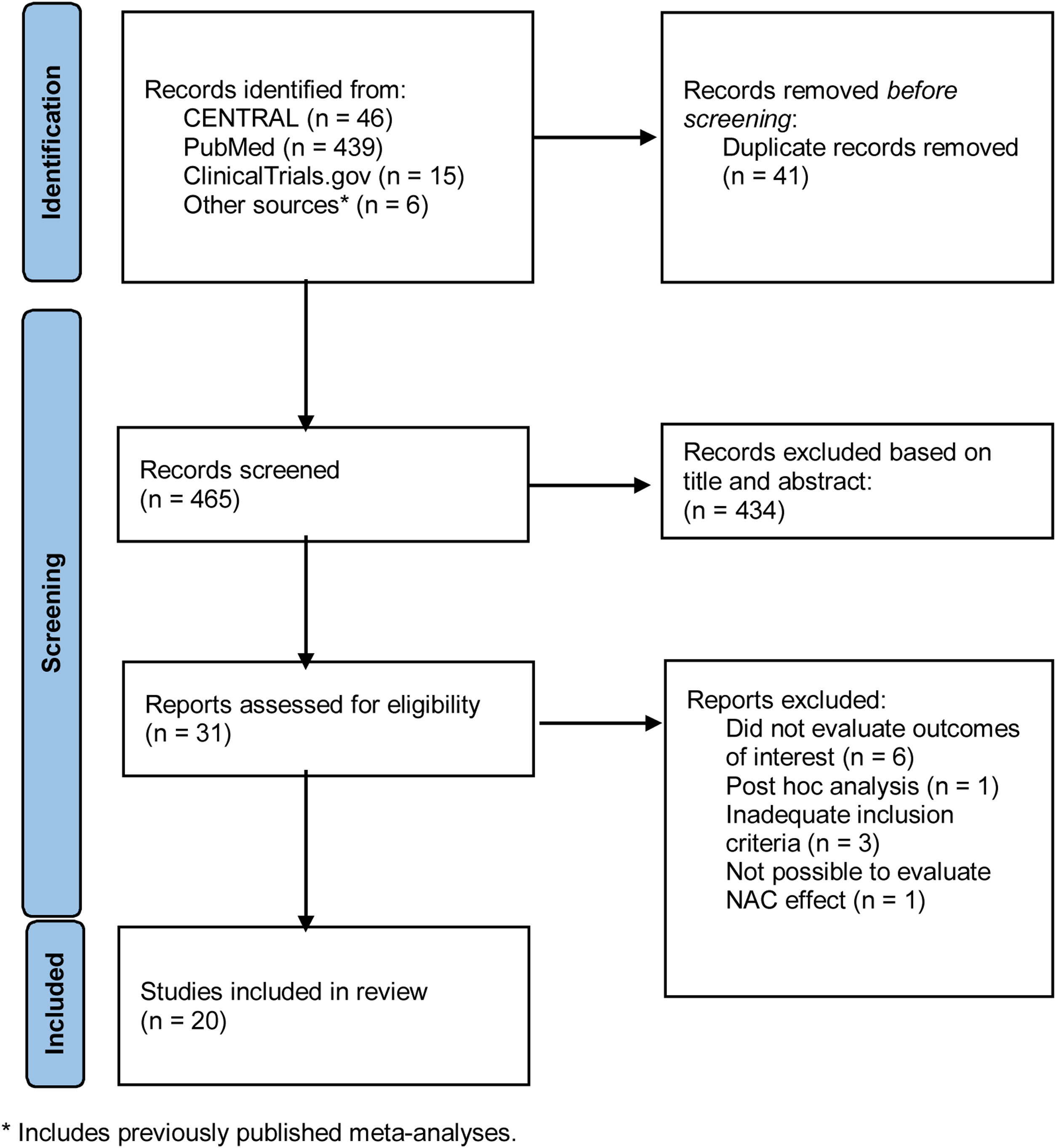
ResultsStudy CharacteristicsThe search results initially identified 465 references, after duplicates removal, we excluded 434 references based on title and abstract and reviewed the remaining 31 full texts for possible inclusion, and further excluded 12 studies. The reasons of exclusion are listed in Fig. 1.
The twenty studies included in the review represented a total of 4044 patients (2016 treatments/2028 placebo). Appendix Tables 3–5 show the characteristic of each study. Information about patients is reported considering data at baseline.
A total of seven studies27–33 evaluated the use of NAC in patients with symptoms of CB as entry criterion. When attainable mean FEV1/FVC ratio was >0.7 in these studies.
Three studies34–36 explored the role of NAC in patients with chronic bronchitis and spirometry confirmed COPD. The remaining 10 studies examined the use of NAC in patients with COPD with documented airflow obstruction with or without symptoms of chronic bronchitis.37–46 Three studies reported spirometry data indicating a mixed population of patients with COPD and patients with chronic bronchitis/pre-COPD without any way of distinguishing them34–36; these studies have been included in the sensitivity analyses. One study was conducted with three arms36 and only the NAC and placebo arms were used in the review. Study duration ranged from 2 months40 to 36 months.36,39
The mean age of patients ranged from 45 to 71 years. Babolini34 only reported age groups and included patients older than 55 years. All studies, except for Boman27 and Babolini,34 reported patient gender. The proportion of males ranged from 43.1%30 to 93.3%.44 The percentage of current smokers was available for 13 studies and ranged from 17.8%45 to 92.2%.30
Seven studies28,29,31,34,35,37,42 did not report the percentage or current smokers. Of these, 3 studies31,34,35 reported the total percentage of current and ex-smokers, respectively 64.4%, 88.5% and 100%.
Overall, NAC dosage varied from 400 to 3600mg daily and treatment lasted from two months to three years. Of the 20 studies, three considered a total N-acetylcysteine dosage of 400mg/day.27,28,34 Twelve studies used a total dosage of 600mg/day29,31–33,35–37,39,41–43,46 and three of 1200mg/day.30,44,45 One study40 used a dosage of 3600mg/day and another38 considered two intervention groups with NAC dosage of 600mg/day and 1200mg/day, in combination with propolis. In the COPD subgroup, five studies used a dose of 600mg and four studies a dose of 1200mg/1800mg (three studies used a dose of 1200mg and one study a dose of 1800mg). In the CB/pre-COPD subgroup, one study used a dose of 200/300mg, two a dose of 400mg, two a dose of 600mg and one used a dose of 1200mg (Appendix Table 5).
Evaluation of NAC Versus Placebo on ExacerbationsTotal Number of Exacerbations in Patients who Completed the StudyThirteen studies reported results on total number of exacerbations during the study period.
Seven studies were included in the COPD subgroup38,44–46 representing 1709 patients. Definition of exacerbations across selected references is presented in the Table 6 of the Appendix.
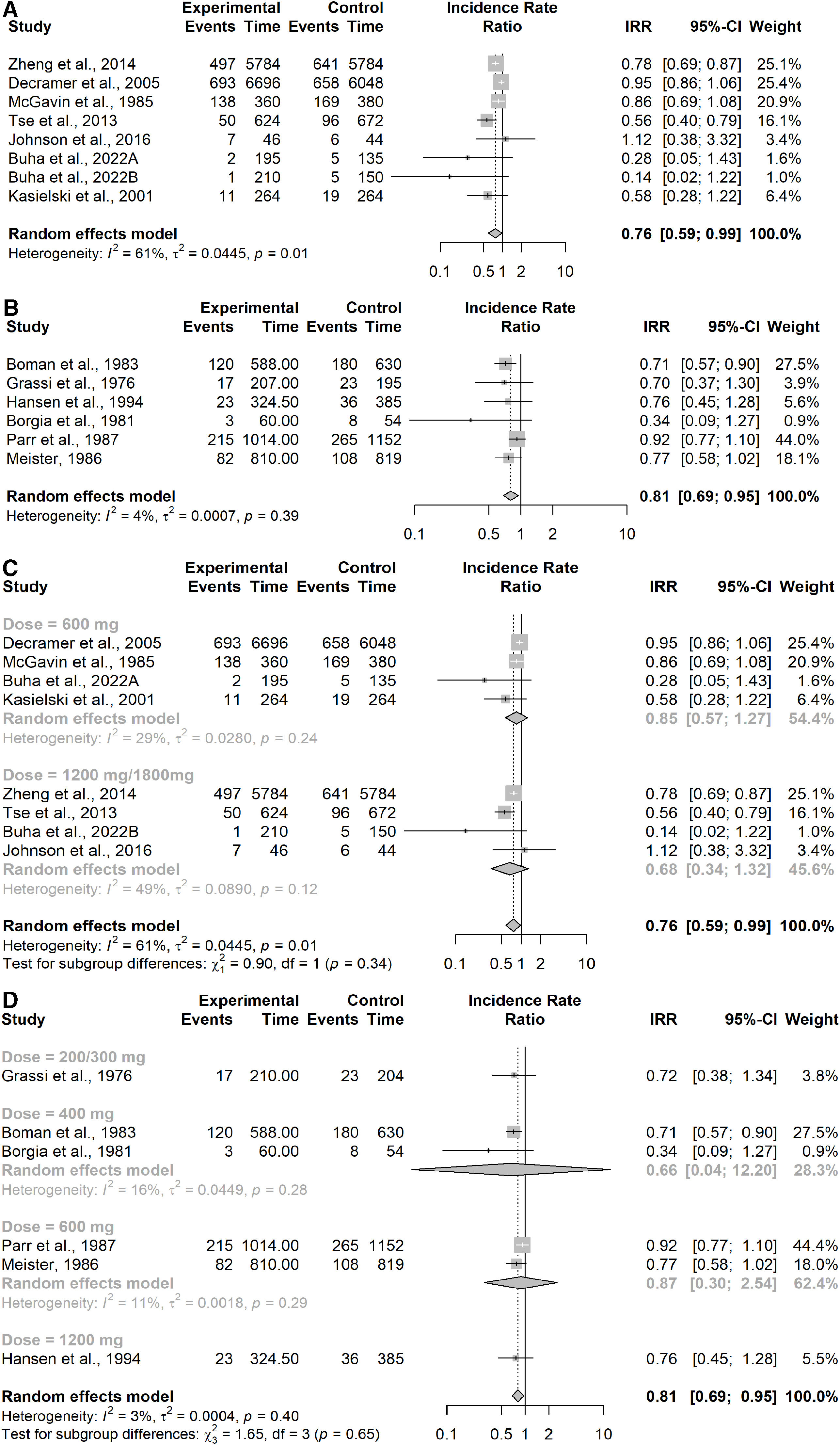
Results showed a significant reduction of the incidence of exacerbations in NAC treated patients compared to placebo (IRR=0.76; 95% confidence interval (CI) 0.59–0.99, patients=1709). Moderate to substantial heterogeneity between studies was detected (I2=61%; p=0.01), Fig. 2A.
Forest Plot from meta-analysis carried out (A) in studies assessing the incidence of exacerbations in the COPD subgroup; (B) in studies assessing the incidence of exacerbations in the CB/pre-COPD subgroup; (C) in studies assessing the incidence of exacerbations in the COPD subgroup by dose level; (D) in studies assessing the incidence of exacerbations in the CB/pre-COPD subgroup by dose level.
Six studies were in the CB/pre-COPD subgroup27–32 representing 962 patients and showed a significant effect of NAC in reducing the incidence of exacerbations compared to placebo (IRR=0.81; 95% CI 0.69–0.95). I2 and Chi-squared test suggests low heterogeneity for this subgroup of studies (I2=4%; p=0.39), confirmed by 95% prediction interval for the pooled IRR (0.67; 0.98), Fig. 2B.
The sensitivity analyses performed by study duration showed consistent results in both subgroups (≤5 months of duration vs >5 months) considering the three additional studies34–36 showed a substantial increase in heterogeneity (CB/pre-COPD: I2=93%; p<0.01, prediction interval 0.31–1.88; COPD: I2=93%; p<0.01, prediction interval 0.29–1.93) and resulted in non-significant difference between patients treated with NAC and placebo but close to favoring NAC over placebo (CB/pre-COPD: IRR=0.76; 95% CI 0.56–1.05, patients=1656; COPD: IRR=0.74; 95% CI 0.54–1.03, patients=2403). This result was influenced by the study by Schermer et al.36 which was the only trial finding a significantly higher number of exacerbations in the NAC group compared with the placebo group.
The sensitivity analysis performed on both subgroups by duration showed consistent results in both subgroups; studies with duration less or equal to 5 months and studies with duration higher than 5 months.
An additional sensitivity analysis was performed by study duration showing consistent results in both subgroups (≤5 months of duration vs >5 months) by dose (Appendix Table 7) showed consistent results across different dose levels in both subgroups (Fig. 2C for the COPD subgroup and Fig. 2D for the CB/pre-COPD).
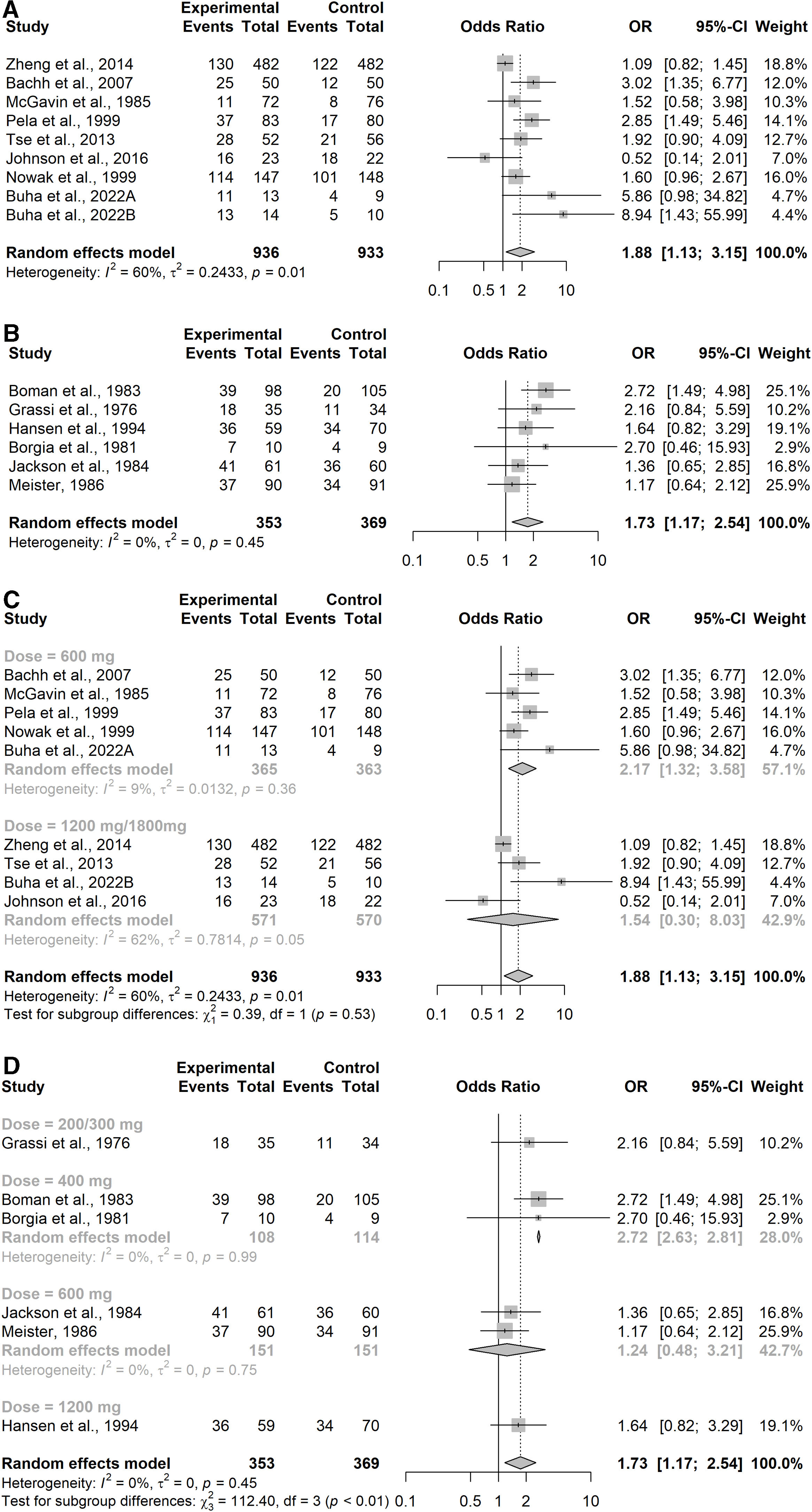
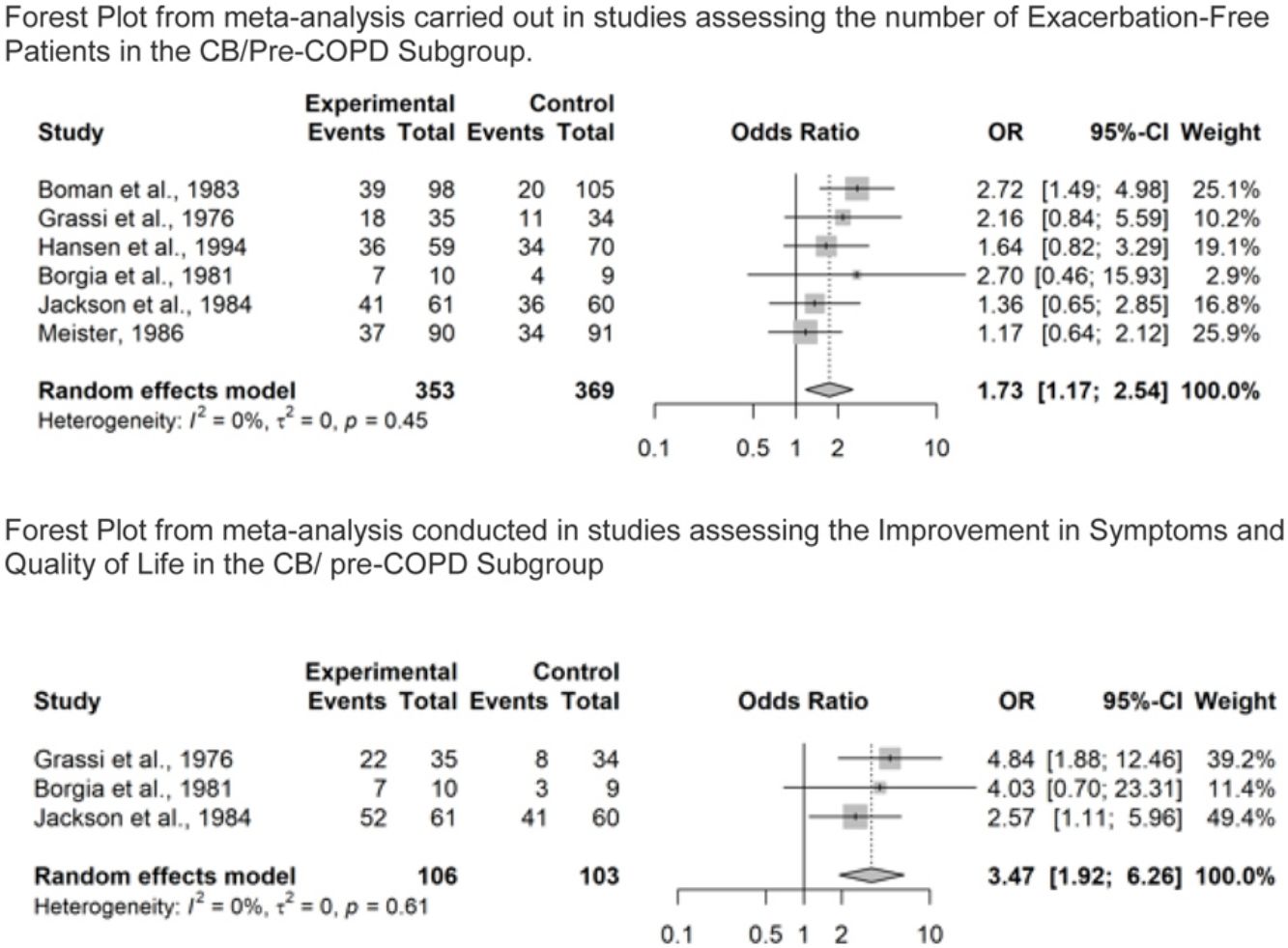
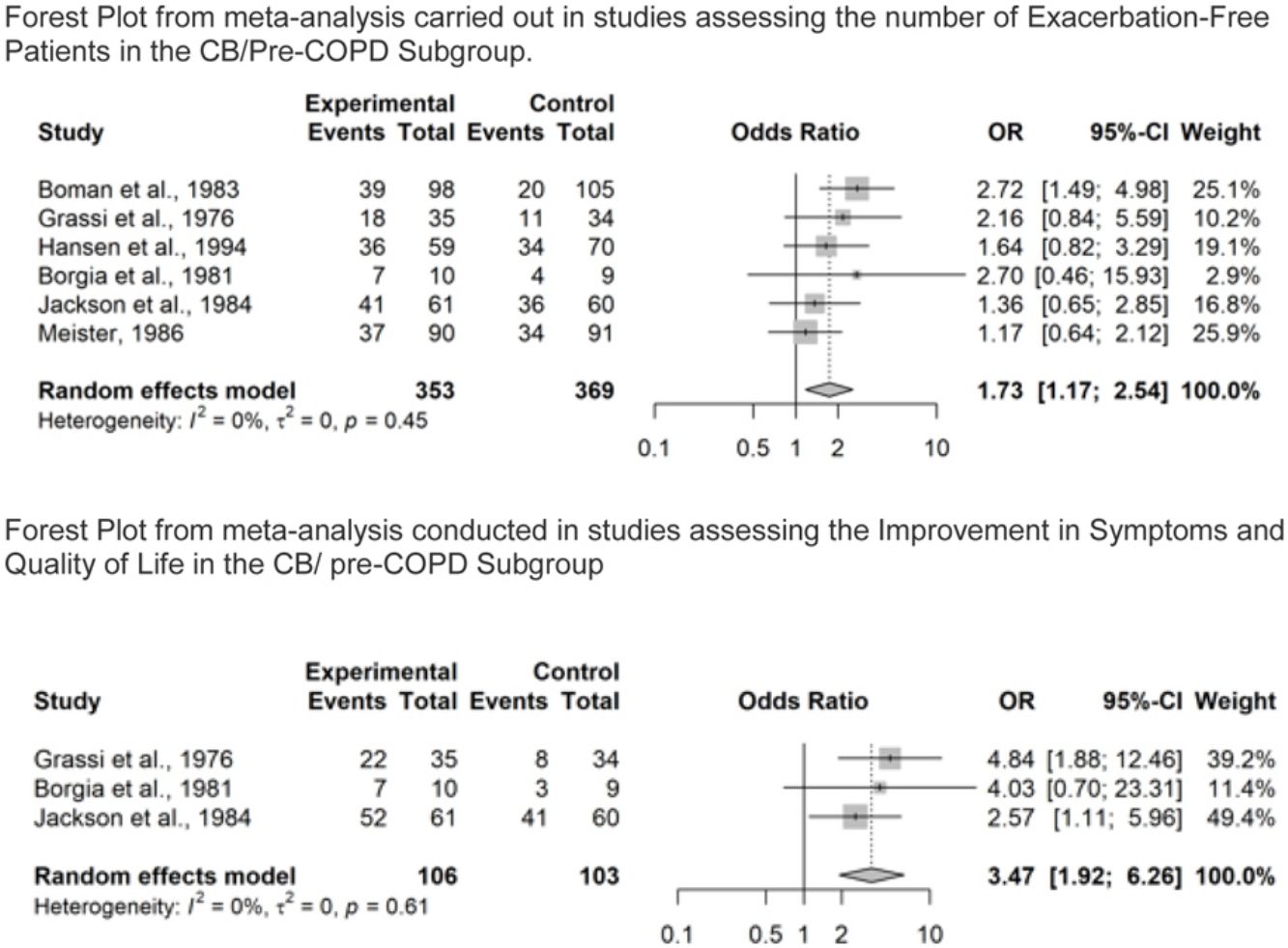
Number of Patients Without Exacerbations During the Study PeriodOverall, seventeen studies reported results on number of patients without exacerbations during the study period. Of these, eight studies were in the COPD subgroup37,38,40,42–46 representing 1896 patients. Results showed that COPD patients treated with NAC were significantly more likely to be exacerbation-free (OR=1.88, 95% confidence interval (CI) 1.13–3.15), as shown in Fig. 3A. Moderate to substantial heterogeneity was detected (I2=60%; p=0.02). In particular, the point estimate was favoring placebo over NAC in one study.40 Six studies were in the CB/pre-COPD subgroup27,33 representing 722 patients, and results showed that patients treated with NAC were significantly more likely to be exacerbation-free (OR=1.73, 95% confidence interval (CI) 1.17–2.54), Fig. 3B.
Forest Plot from meta-analysis carried out (A) in studies assessing the number of exacerbation-free patients in the COPD subgroup; (B) in studies assessing the number of exacerbation-free patients in the CB/pre-COPD subgroup; (C) in studies assessing the number of exacerbation-free patients in the COPD subgroup by dose level; (D) in studies assessing the number of exacerbation-free patients in the CB/pre-COPD subgroup by dose level.
Results did not highlight heterogeneity between studies (I2=0%; p=0.45).
Sensitivity analyses performed on both subgroups considering the three additional studies34–36 showed a moderate increase in heterogeneity (CB/pre-COPD: I2=59%; p=0.01, prediction interval 0.78–4.23; COPD: I2=72%; p<0.01, prediction interval 0.56–6.09) and confirmed significant protective effect of NAC on the risk of experiencing an exacerbation (CB/pre-COPD: OR=1.81; 95% CI 1.25–2.64, patients=1416; COPD: OR=1.85; 95% CI 1.22–2.81, patients=2563).
Additional sensitivity analysis was performed on both subgroups by duration showing consistent results in both subgroups; studies with duration less or equal to 5 months and studies with duration higher than 5 months.
The sensitivity analysis by dose conducted on both subgroups (Appendix Table 8) showed consistent results across different dose levels in both of the subgroups (Fig. 3C for the COPD subgroup and Fig. 3D for the CB/pre-COPD).
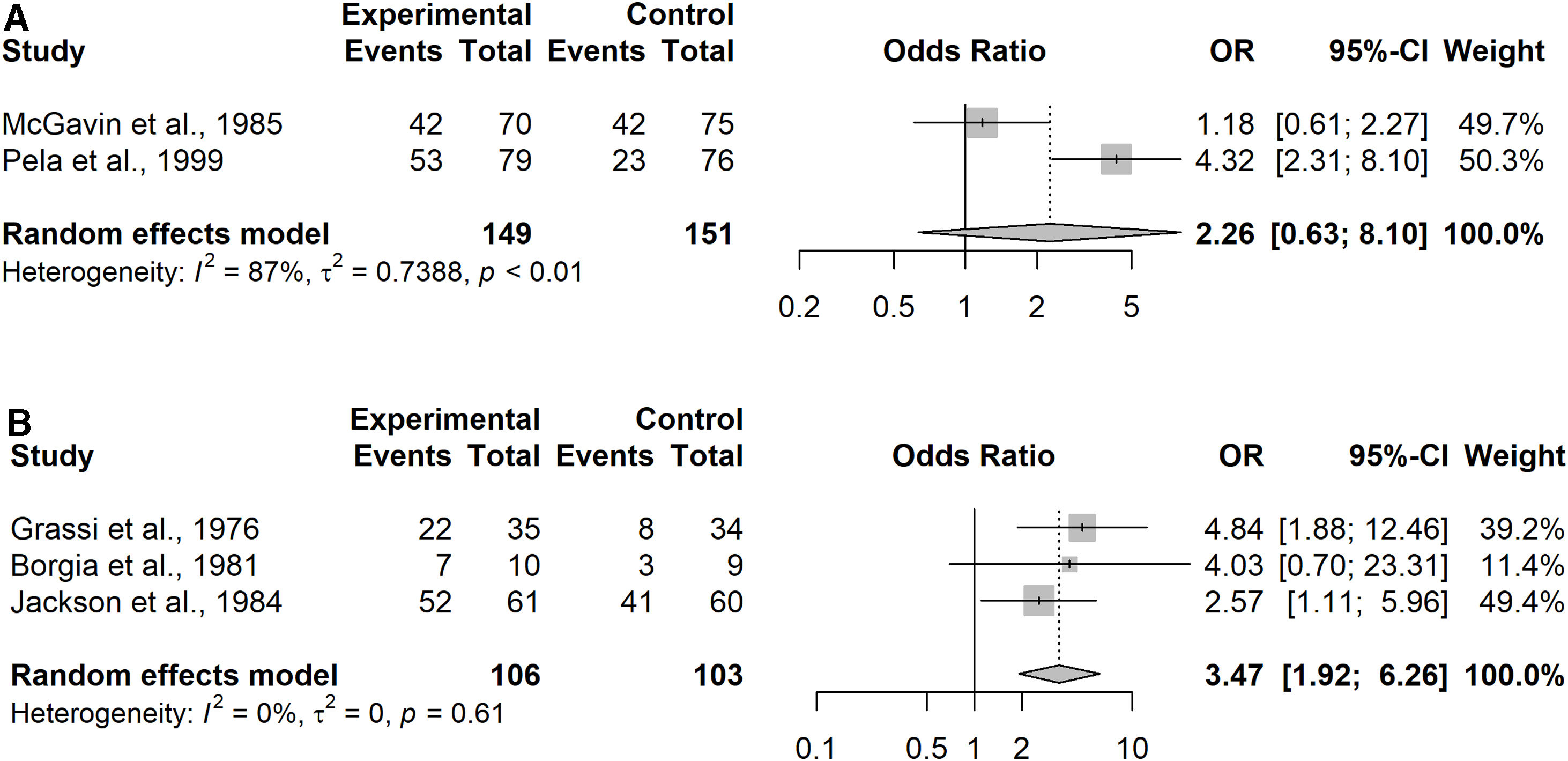
Evaluation of NAC Versus Placebo on Improvements of Respiratory Symptoms and Quality of LifeFive studies reported respiratory symptoms and quality of life (Appendix Table 9). Due to the heterogeneity of available data, improvement was assessed by the criteria adopted in the studies identified, as detailed in Appendix Table 10. Two studies were conducted in the COPD subgroup43,46 representing 300 patients. Data were however not available for 3 subjects in McGavin et al.46 (2 in NAC group and 1 in placebo group) and for 8 subjects in Pela et al.43 (4 in NAC group and 4 in placebo group).
Given the heterogeneity of the tools utilized to assess the symptom burden, the pooled odds ratio favored NAC over placebo, but without reaching significance (OR=2.26, 95% confidence interval (CI) 0.63–8.10, patients=300; Analysis 1.2). Substantial heterogeneity was detected (I2=87%; p<0.01), Fig. 4A.
Three studies were conducted in the CB/pre-COPD subgroup28,29,33 representing 209 patients. Patients treated with NAC were significantly more likely to experience an improvement in symptoms and/or quality of life over the study period compared to placebo (OR=3.47; 95% confidence interval (CI) 1.92–6.26). Results did not highlight important heterogeneity in the studies (I2=0%; p=0.61), Fig. 4B.
Sensitivity analysis performed on both subgroups considering the additional study34 showed no impact on heterogeneity in the CB/pre-COPD subgroup but confirmed substantial heterogeneity in the COPD subgroup (CB/pre-COPD: I2=0%; p=0.75; COPD: I2=83%; p<0.01). Results confirmed the significant association of NAC with the likelihood of experiencing an improvement in symptoms and/or quality of life (CB/pre-COPD: OR=3.93; 95% CI 2.97–5.22, patients=820; COPD: OR=2.82; 95% CI 1.25–6.38, patients=911).
DiscussionResults of the present meta-analysis show that the benefit provided by NAC, when exacerbations are considered, was similar in both study subgroups: COPD and CB/pre-COPD. In addition, the symptom burden is decreased and the quality of life is improved in the CB/pre-COPD group, with a numerical trend in the same direction in COPD patients. Heterogeneity between studies has been observed in these analyses as detailed below.
At variance with prior pooled analyses on NAC efficacy in smoking related diseases10,47,48 here we evaluated the available data focusing on exacerbations and symptoms and in two different subpopulation COPD and in CB/pre-COPD. The latter is of particular clinical interest3 given that, while NAC has been previously investigated mainly for its effects in exacerbation prevention in COPD, whereas neither the effects of NAC on symptoms in COPD nor the efficacy data of NAC in CB/pre-COPD have ever undergone prior meta-analysis assessments. When focusing on exacerbations the benefit provided by NAC treatment has been shown in both subgroups of studies, COPD and CB/pre-COPD which provided similar results. The former findings are of particular interest and novelty as the efficacy of treatments on respiratory symptoms in CB patients (particularly non selected for airflow limitation) has been seldom carried out, despite the clinical relevance of this information.
It is increasingly clear that patients with a history of exposure to cigarette smoke or other environmental pollutants may have a substantial disease burden with lung pathology and respiratory impairment even in the absence of airflow limitation, as detected by spirometry.49 Not all of these patients will develop airflow limitation, but many will have considerable respiratory morbidity and a comparable prognosis to those with classical, spirometry defined COPD.50
In our meta-analysis, benefit in respiratory symptoms and quality of life has been shown in patients with chronic bronchitis with no documented evidence of airflow limitation. This was also the case in the subgroup of COPD patients when considering additional studies in the sensitivity analysis. However, these results should be interpreted with caution given the variability of the tools used to assess the improvement in respiratory symptoms and quality of life and the limited number of studies reporting this outcome.
Oxidative stress is increased and contributes to the inflammation process in COPD patients especially during exacerbations.2 Reactive oxygen species generation promotes the release of mucins from epithelial cells51,52 which can contribute to the clinical expression of the acute event. NAC with its antioxidant properties may therefore counteract the oxidative process and down-regulate the production of MUC5AC.51–55
Most recent large clinical trials evaluating NAC in COPD suggest a reduced exacerbation rate in the subgroup of patients treated with NAC. In the BRONCUS trial,39 although no effect on the rate of decline of forced expiratory volume in the first second of expiration (FEV 1) was observed, NAC decreased the number of COPD exacerbations in patients not taking inhaled corticosteroids, and secondary analyses also suggested an effect on hyperinflation. The PANTHEON study evaluated the effect of NAC in addition to existing individual therapy in 1006 patients with stable, moderate-to-severe COPD.45 Trial results underlined the greater effect of NAC in reducing the number of exacerbations especially in patients with moderate disease (GOLD II), thus suggesting the use of NAC at an earlier stage can help prevent exacerbations and long term consequences.
As for the dose related response of NAC, in line with other assessments10,12,47 which have used different selection criteria, our meta-analysis showed no clear dose response on the effect of NAC on exacerbations.
The study has limitations. Moderate to substantial heterogeneity has also been noted among studies in the COPD subgroup. These studies varied substantially in term of duration of therapy, NAC dose, demographics, and number of participants; therefore, results should be interpreted with caution and further analyses are needed to investigate the causes of the heterogeneity. Heterogeneity was generally lower or not present in the CB/pre-COPD subgroup of studies. Older studies are more difficult to evaluate in term of publication bias, which lead to a lower level of confidence in the overall treatment effect estimate. One of the limitations is related to the fact that in some of the older studies the population was recruited based on CB while lung function was not an inclusion criteria. Thus, patients from these studies can belong to the CB/pre-COPD or the COPD+CB subgroups. Finally, available data did not allow to include in the analyses two important markers for exacerbations and symptom management such as inhaled treatment (specifically inhaled corticosteroids) and eosinophil count.
ConclusionThe findings from this meta-analysis represent additional evidence of NAC potential benefit in the treatment of COPD and CB/pre-COPD patients in preventing occurrence of exacerbations and improvement in patients’ symptoms and quality of life.
Furthermore, it provides additional evidence in the potential benefit of N-acetylcysteine in CB/pre-COPD patient population.
FundingStatistical and editorial support was provided by Zambon S.p.A.
Conflicts of InterestCristina Aljama has received speaker fees from FAES farma, Zambon, GSK and CSL Behring.
Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, BEAM Therapeutics, Chiesi, GlaxoSmithKline, CSL Behring, Ferrer, Inhibrx, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon, Zentiva and Grifols and research grants from Grifols.
Marco Contoli declares research grants from Chiesi and GSK; personal payments for lectures, presentations or educational events from Chiesi, Astrazeneca, GSK, Novartis, Zambon, Sanofi, Alk Abello; personal payments for participation on Advisory Board from Chiesi, Astrazeneca, Gsk, Alk Abello, Sanofi.
Alberto Papi has received personal fees from AstraZeneca, Avillion, Chiesi Farmaceutici, Chiesi Italia, Dompé, Galvanize, Elpen, GSK, Menarini, Moderna, Mundipharma, Novartis, Orient Europharma, Roche, Sanofi, Zambon, grants for research form Chiesi, Astrazeneca, GSK, Sanofi.
Amal Mawass, Franco Alfano, Tommaso Bigoni, Lorenzo Mancini, Federico Baraldi declare to have no conflict of interest directly or indirectly related to the manuscript contents.
The authors thank Elisa Veratelli for the editorial support.