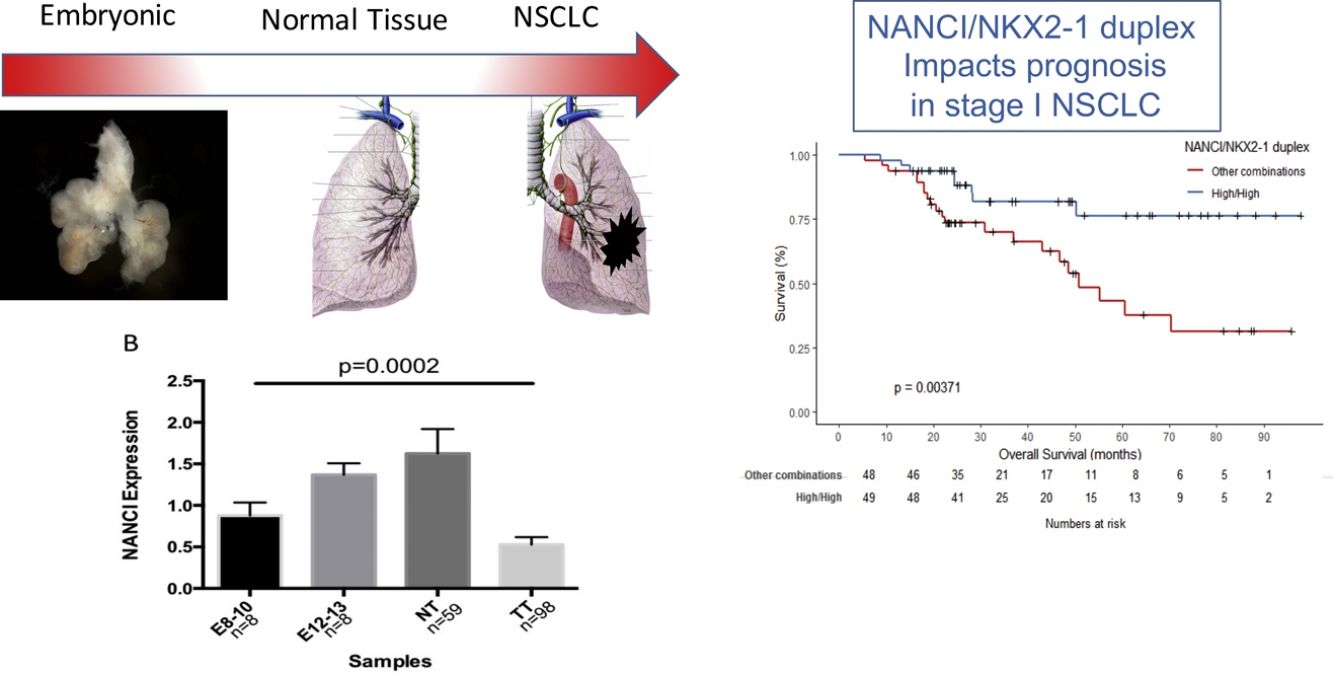
NANCI, an intergenic long non-coding RNA (lncRNA) is essential for buffering NKX2-1 expression during embryonic development and in adult tissue. We analyzed NANCI and NKX2-1 in human lung embryonic samples and adult lung tissues and evaluated their potential as prognostic markers in stage I non-small cell lung cancer (NSCLC).
Methods and resultsNANCI and NKX2-1 expression was assessed by TaqMan assays in 18 human embryonic samples from 8 to 13 weeks, 59 non-tumoral (NT) lung tissue samples, and 98 stage I NSCLC tumor samples. NANCI and NKX2-1 expression in embryonic and NSCLC samples were downregulated in comparison to adult NT tissue. Patients with low expression of NANCI had shorter disease-free survival (DFS) and overall survival (OS) than those with high levels (47.6 vs 69.3 months, P=0.032 and 57.7 vs 77.6 months, P=0.021, respectively). When the expression levels of NANCI and NKX2-1 were evaluated in combination, four groups were identified (high NANCI/high NKX2-1, low NANCI/high NKX2-1, high NANCI/low NKX2-1 and low NANCI/low NKX2-1) with differential impact on DFS (P=0.042) and OS (P=0.024). Interestingly, the high NANCI/high NKX2-1 duplex group had longer DFS and OS than the other three groups (71.25 vs 46.3 months, P=0.009 and 81.3 vs 56.1 months, P=0.004, respectively). In the multivariate analysis, the high NANCI/high NKX2-1 duplex was identified as an independent prognostic factor for longer DFS (HR 0.346, 95% CI, 0.169–0.709; P=0.004) and OS (HR 0.309, 95% CI, 0.121–0.786; P=0.014).
ConclusionsNANCI and the NANCI-NKX2-1 duplex impacts prognosis in stage I NSCLC patients.
NANCI, un ARN intergénico largo no codificante (lncRNA) es esencial para regular la expresión de NKX2-1 durante el desarrollo embrionario y en el tejido adulto. Analizamos la expresión de NANCI y NKX2-1 en muestras embrionarias de pulmón humano y tejidos pulmonares adultos, y evaluamos su potencial como marcadores pronósticos en el cáncer de pulmón de células no pequeñas (CPCNP) en estadio i.
Métodos y resultadosLa expresión de NANCI y NKX2-1 se evaluó mediante ensayos TaqMan® en 18 muestras embrionarias humanas de 8 a 13 semanas, 59 muestras de tejido pulmonar no tumoral (NT) y 98 muestras de tumor de CPCNP en estadio i. La expresión de NANCI y NKX2-1 en muestras embrionarias y NSCLC se encontraba reducida en comparación con el tejido NT adulto. Los pacientes con baja expresión de NANCI tuvieron una supervivencia libre de enfermedad (SLE) y una supervivencia general (SG) más cortas que aquellos con niveles altos (47,6 frente a 69,3 meses; p=0,032 y 57,7 frente a 77,6 meses; p=0,021, respectivamente). Cuando se evaluaron los niveles de expresión de NANCI y NKX2-1 combinados se identificaron 4 grupos (NANCI alto/NKX2-1 alto, NANCI bajo/NKX2-1 alto, NANCI alto/NKX2-1 bajo y NANCI bajo/NKX2-1 bajo) con impacto variable en la SLE (p=0,042) y la SG (p=0,024). Curiosamente, la combinación de NANCI alto/NKX2-1 alto presentó unas SLE y SG más largas que los otros 3 grupos (71,25 frente a 46,3 meses; p=0,009 y 81,3 frente a 56,1 meses; p=0,004, respectivamente). En el análisis multivariante, la combinación de NANCI alto/NKX2-1 alto se identificó como un factor de pronóstico independiente para una SLE más larga (HR: 0,346; IC 95%: 0,169-0,709; p=0,004), al igual que la SG (HR: 0,309; IC 95%: 0,121-0,786; p=0,014).
ConclusionesNANCI y la combinación de NANCI-NKX2-1 afecta al pronóstico de los pacientes con CPCNP en estadio i.
The cornerstone of treatment of early stage (I–II) non-small cell lung cancer (NSCLC) is surgery since the surgical removal of the tumor is considered potentially curative in these patients.1 The addition of adjuvant treatment after surgery (cisplatin-based chemotherapy) is clear for patients with N1 disease but more controversial in those with N0 disease.2 The identification of prognostic markers of high risk is critical to anticipate outcomes and to identify stage I patients likely to benefit from adjuvant treatment.
Long non-coding RNAs (lncRNAs) are RNA transcripts longer than 200 nucleotides with no protein-coding capability.3 Their expression pattern is tissue- and cancer-type-specific,4 making them promising biomarkers. In NSCLC, several lncRNAs play a leading role in carcinogenesis where some of them act as biomarkers for treatment response and prognosis.5 Some lncRNAs can also be regulated by transcription factors, such as TP53,6 which is frequently mutated in NSCLC patients.
NKX2-1 gene (also known as TTF-1) plays an essential role in lung, thyroid and forebrain development during embryogenesis7 and complete loss of NKX2-1 results in the failed development of trachea or lung branching.8 Embryogenesis and cancer share a number of cellular and molecular features.9,10 However, as opposed to embryogenesis, cancer is characterized by dysregulated and uncontrolled cell proliferation. The expression of NKX2-1, a critical gene for lung development, has been widely studied in lung cancer where the absence of NKX2-1 expression defines a subgroup of NSCLC patients with dismal prognosis, particularly in early stage patients.11,12 The LncRNA NANCI (NKX2-1 associated noncoding intergenic RNA) is a 2.5-kb lncRNA spatially located near the critical transcription factor NKX2-1.13 NANCI knockdown resulted in decreased expression of NKX2-1,14 indicating that NANCI regulates NKX2-1 expression.
We hypothesized that NANCI and its regulated gene NKX2-1 are embryonic genes that become dysregulated during oncogenesis and could play a promising role as prognostic biomarkers in NSCLC. In order to test this hypothesis, we analyzed NANCI and NKX2-1 expression in human embryonic lung samples and in adult normal lung and stage I NSCLC tissue samples and correlated our findings with overall survival (OS) and disease-free survival (DFS).
Materials and methodsPatient samplesFrom June 2007 to July 2014, tumor and paired normal tissue samples were prospectively collected from 98 adult patients diagnosed with stage I NSCLC who underwent complete surgical resection at Hospital Clínic de Barcelona (Barcelona, Spain). None of the patients received neoadjuvant or adjuvant treatment. Tissue samples were immediately immersed in RNALater® (Ambion) and stored at −80°C until processing. Clinical data were recorded on admission: age, gender, smoking history, preoperative pulmonary function tests (PFT), chronic obstructive pulmonary disease (COPD), Eastern Cooperative Oncology Group (ECOG) performance status (PS), clinical and postoperative staging according to the TNM 7th edition, which was used during diagnostic workout and treatment decision, type of surgical resection and pathological findings (histological subtype, and the presence of emphysema). The data referring to the TNM 8th edition are available in supplementary file 1. The mutational status of TP53 and K-RAS was assessed in 80% of patients, and EGFR mutational status was assessed in adenocarcinoma (ADK) patients, as previously described.15 Approval for the study was obtained from the Clinical Research Ethics Committee of the Hospital Clínic de Barcelona, and written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Human embryonic samplesEighteen spontaneously aborted embryos and fetuses were donated with written informed consent to the Body Donation Service of the Human Anatomy and Embryology Unit of the School of Medicine of University of Barcelona for morphological and molecular studies. The samples included lungs from the 8th to the 13th week of development. Lung samples were obtained under control of an Olympus stereo microscope SZ61.
RNA extraction and gene expression analysisTotal RNA was isolated from frozen tissue using TriZol® Reagent (Life Technologies) according to the manufacturer's protocol. RNA from samples was quantified using a NanoDrop ND-1000 Spectrophotometer (Fisher Scientific, Madrid, Spain). The High Capacity cDNA Reverse Transcription Kit® (Life Technologies) was used to obtain cDNA from 500ng of total RNA as per manufacturer's protocol. NKX2-1 mRNA expression levels were quantified using a TaqMan Gene Expression assay (Hs00968940_m1) in a 7500 Real-Time PCR System (Life Technologies). NANCI expression levels were quantified using a custom Taqman Expression assay according to geneBank KF856952.1 accession number.16 The Taqman assay was designed to detect specifically NANCI expression since was designed in the 51nt in the 5′ region that not overlap with any transcript of SFTA3 gene. Relative expression levels were calculated by 2−Ct method using 18S as endogenous control.
Statistical analysesThe primary endpoints of the study were OS and DFS. OS was defined as the time from surgery to either death from any cause or last follow-up. DFS was defined as the time from surgery to either recurrence or death, or last follow-up. Optimal cut-offs of NANCI and NKX2-1 expression data for OS and DFS were assessed by means of maximally selected log-rank statistics using the Maxstat package (R statistical package, v. 2.8.1, Vienna, Austria) and Kaplan–Meier curves were drawn and compared by means of a log-rank test. All factors with P≤0.1 in the univariate analysis were included in the Cox multivariate regression analyses for DFS and OS. Student t-test or Mann–Whitney U test, as appropriate, were used for comparisons between two groups or ANOVA when more than 2 groups were compared. Pearson correlation was used to compare NANCI and NKX2-1 expression. All statistical analyses were performed using PASW Statistics 21 (SPSS Inc.) and R v2.8.1. The level of significance was set at P≤0.05.
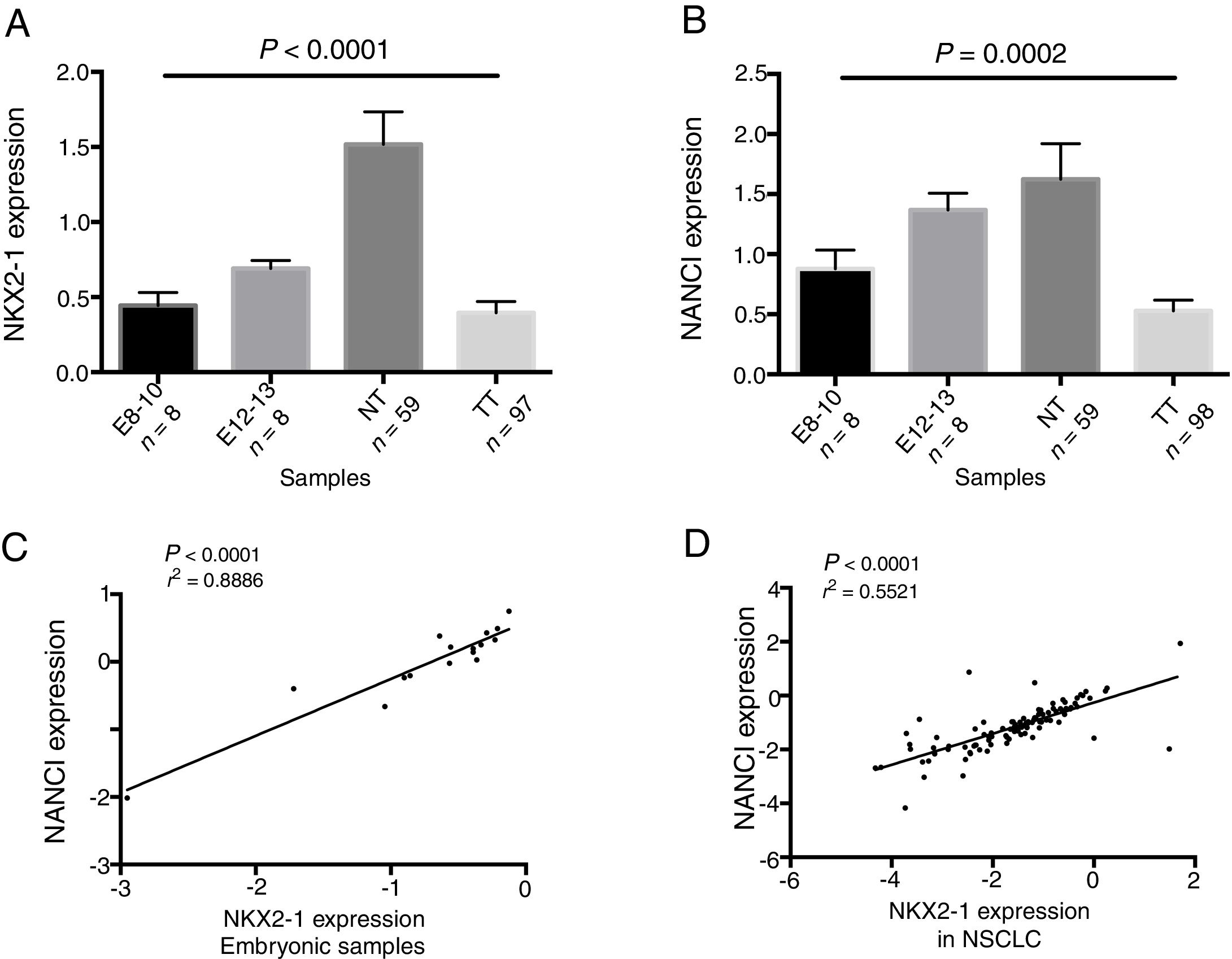
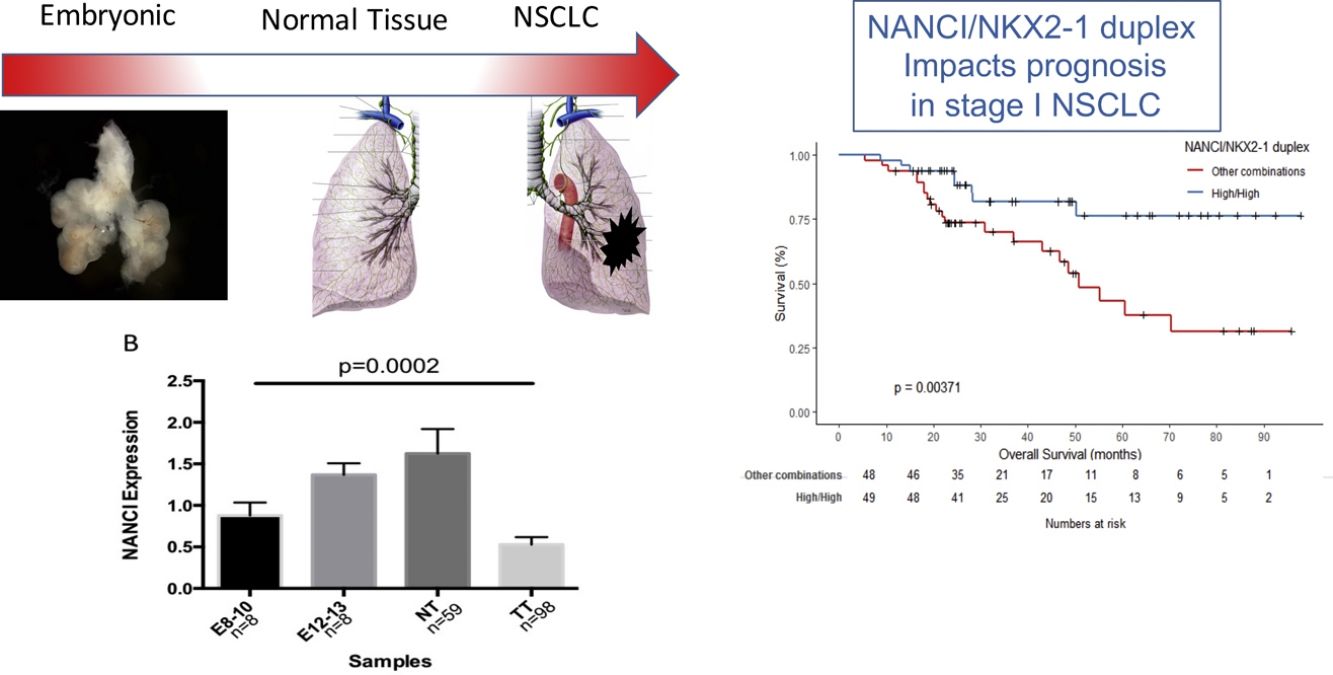
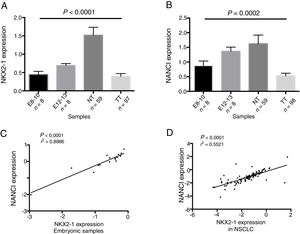
ResultsNANCI and NKX2-1 expression in human lung embryogenesis and lung cancerThe expression of NANCI and NKX2-1 was assessed in human embryonic lung tissue from embryos of 8–13 weeks of development and adult normal lung and lung cancer tissues (Fig. 1A and B). In embryonic samples, the expression of NANCI and NKX2-1 was highly correlated (r2=0.8886, P<0.0001; Fig. 1C). When we compared the embryonic expression longitudinally from the 8th to the 13th week, significant changes in expression were observed. The expression of both NANCI and NKX2-1 increased as the lung became more differentiated, with the highest levels found in the normal adult tissue samples (P=0.0002 and P<0.0001, respectively). However, a significant reduction in overall levels was observed in lung tumor tissue compared to normal adult tissue (P<0.0001). A positive correlation between NANCI and NKX2-1 was also observed in tumor tissue (r2=0.5521, P<0.001; Fig. 1D).
PatientsTable 1 displays the characteristics of the 98 stage I NSCLC patients included in the study. The majority were male (74.5%), with a mean age of 67.3 years (range, 38–84). Eighty-one patients (83%) had ECOG PS 1 and eighty-four patients (85%) were current or former smokers. Pulmonary function tests showed a mean FEV1/FVC (%) ratio of 64.25±11.11, confirming COPD diagnosis in 68% of all patients, with a mean carbon monoxide diffusing capacity (DLCO%) of 73% (range, 35–101%).
Main patient characteristics, univariate P-values (log-rank test) for disease-free survival (DFS) and overall survival (OS) and the mean±standard deviation (SD) of NANCI expression for each clinical characteristic compared by t-test or ANOVA test.
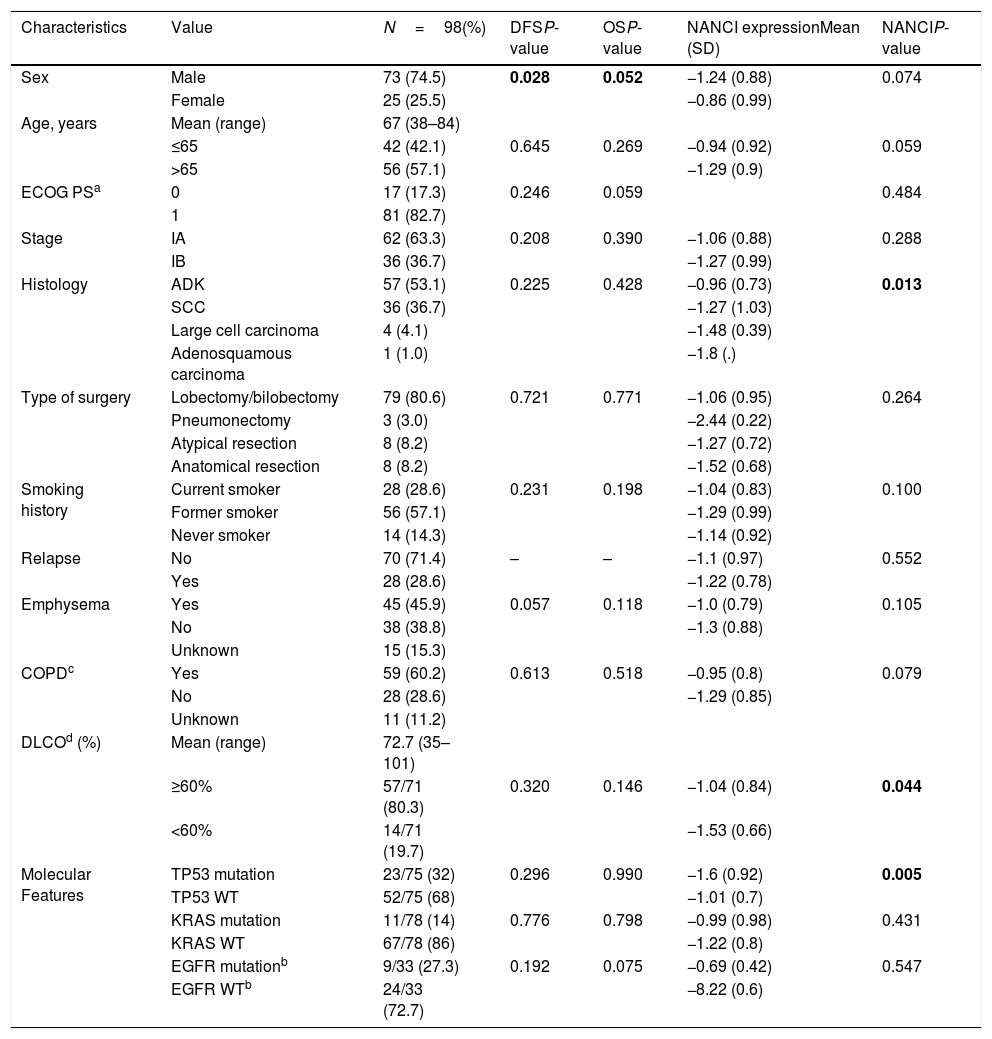
| Characteristics | Value | N=98(%) | DFSP-value | OSP-value | NANCI expressionMean (SD) | NANCIP-value |
|---|---|---|---|---|---|---|
| Sex | Male | 73 (74.5) | 0.028 | 0.052 | −1.24 (0.88) | 0.074 |
| Female | 25 (25.5) | −0.86 (0.99) | ||||
| Age, years | Mean (range) | 67 (38–84) | ||||
| ≤65 | 42 (42.1) | 0.645 | 0.269 | −0.94 (0.92) | 0.059 | |
| >65 | 56 (57.1) | −1.29 (0.9) | ||||
| ECOG PSa | 0 | 17 (17.3) | 0.246 | 0.059 | 0.484 | |
| 1 | 81 (82.7) | |||||
| Stage | IA | 62 (63.3) | 0.208 | 0.390 | −1.06 (0.88) | 0.288 |
| IB | 36 (36.7) | −1.27 (0.99) | ||||
| Histology | ADK | 57 (53.1) | 0.225 | 0.428 | −0.96 (0.73) | 0.013 |
| SCC | 36 (36.7) | −1.27 (1.03) | ||||
| Large cell carcinoma | 4 (4.1) | −1.48 (0.39) | ||||
| Adenosquamous carcinoma | 1 (1.0) | −1.8 (.) | ||||
| Type of surgery | Lobectomy/bilobectomy | 79 (80.6) | 0.721 | 0.771 | −1.06 (0.95) | 0.264 |
| Pneumonectomy | 3 (3.0) | −2.44 (0.22) | ||||
| Atypical resection | 8 (8.2) | −1.27 (0.72) | ||||
| Anatomical resection | 8 (8.2) | −1.52 (0.68) | ||||
| Smoking history | Current smoker | 28 (28.6) | 0.231 | 0.198 | −1.04 (0.83) | 0.100 |
| Former smoker | 56 (57.1) | −1.29 (0.99) | ||||
| Never smoker | 14 (14.3) | −1.14 (0.92) | ||||
| Relapse | No | 70 (71.4) | – | – | −1.1 (0.97) | 0.552 |
| Yes | 28 (28.6) | −1.22 (0.78) | ||||
| Emphysema | Yes | 45 (45.9) | 0.057 | 0.118 | −1.0 (0.79) | 0.105 |
| No | 38 (38.8) | −1.3 (0.88) | ||||
| Unknown | 15 (15.3) | |||||
| COPDc | Yes | 59 (60.2) | 0.613 | 0.518 | −0.95 (0.8) | 0.079 |
| No | 28 (28.6) | −1.29 (0.85) | ||||
| Unknown | 11 (11.2) | |||||
| DLCOd (%) | Mean (range) | 72.7 (35–101) | ||||
| ≥60% | 57/71 (80.3) | 0.320 | 0.146 | −1.04 (0.84) | 0.044 | |
| <60% | 14/71 (19.7) | −1.53 (0.66) | ||||
| Molecular Features | TP53 mutation | 23/75 (32) | 0.296 | 0.990 | −1.6 (0.92) | 0.005 |
| TP53 WT | 52/75 (68) | −1.01 (0.7) | ||||
| KRAS mutation | 11/78 (14) | 0.776 | 0.798 | −0.99 (0.98) | 0.431 | |
| KRAS WT | 67/78 (86) | −1.22 (0.8) | ||||
| EGFR mutationb | 9/33 (27.3) | 0.192 | 0.075 | −0.69 (0.42) | 0.547 | |
| EGFR WTb | 24/33 (72.7) | −8.22 (0.6) |
Bold values correspond to variables with p < 0.05.
The most frequent type of surgical resection was lobectomy in 79 patients (80.6%). Sixty-two patients (63.3%) had stage IA and 36 stage IB disease. Emphysema was found in 45.9% of patients. ADK was the most frequent histological subtype, accounting for 57 patients (58%), followed by squamous cell carcinoma (SCC) in 36 patients (36.7%). Twenty-nine patients died during follow-up, 17 of whom (58.6%) died after relapse.
NANCI lncRNA expression and clinical–pathological characteristicsNANCI lncRNA was differentially expressed according to histological subtype (P=0.013), with higher levels in ADK than in SCC (P=0.004). NANCI lncRNA was downregulated in patients with DLCO <60% (P=0.044). Patients harboring TP53 mutations had a lower expression of NANCI (P=0.005). No significant differences in NANCI expression were found either in EGFR- or KRAS-mutated patients.
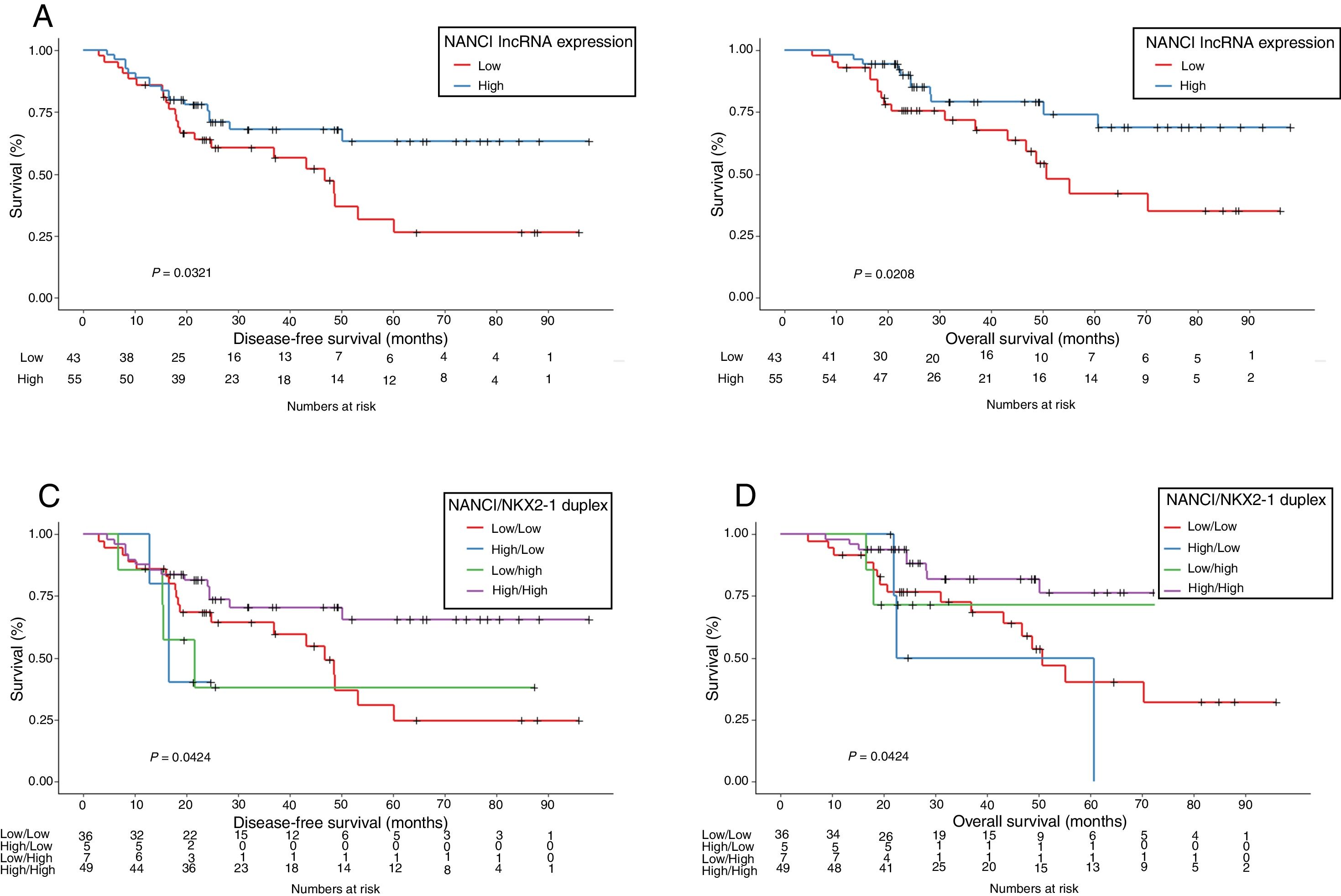
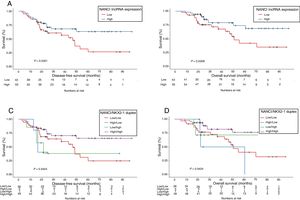
NANCI lncRNA impacts prognosis in stage I NSCLCUsing the cutoff identified by Maxstat package of R, patients were classified as having high (n=55) or low (n=43) NANCI lncRNA expression. With a mean follow-up of 40.4 months (±26.7 months), patients with low expression had shorter mean DFS (47.6 vs 69.3 months; P=0.032) and shorter mean OS (57.7 vs 77.6 months; P=0.021) than those with high expression (Fig. 2A and B).
The prognostic impact of NKX2-1 for the patients included in the present study was also evaluated and is shown in Supplementary Fig. 1A and B. Confirming our previous results in a smaller patient sample,12 low NKX2-1 levels were associated with shorter DFS and OS (P=0.037 and P=0.005, respectively).
NANCI/NKX2-1 duplex and clinical outcomeIn order to evaluate the combined impact of both NANCI and NKX2-1 expression, we classified patients in four groups: 49 (50%) with high NANCI/high NKX2-1; five (5.1%) with high NANCI/low NKX2-1; seven (7.1%) with low NANCI/high NKX2-1; and 36 (36.7%) with low NANCI/low NKX2-1.
Mean DFS was 71.2 months in patients with high NANCI/high NKX2-1, 19 months in those with high NANCI/low NKX2-1, 42.7 months in those with low NANCI/high NKX2-1, and 48 months in those with low NANCI/low NKX2-1 (P=0.042; Fig. 2C).
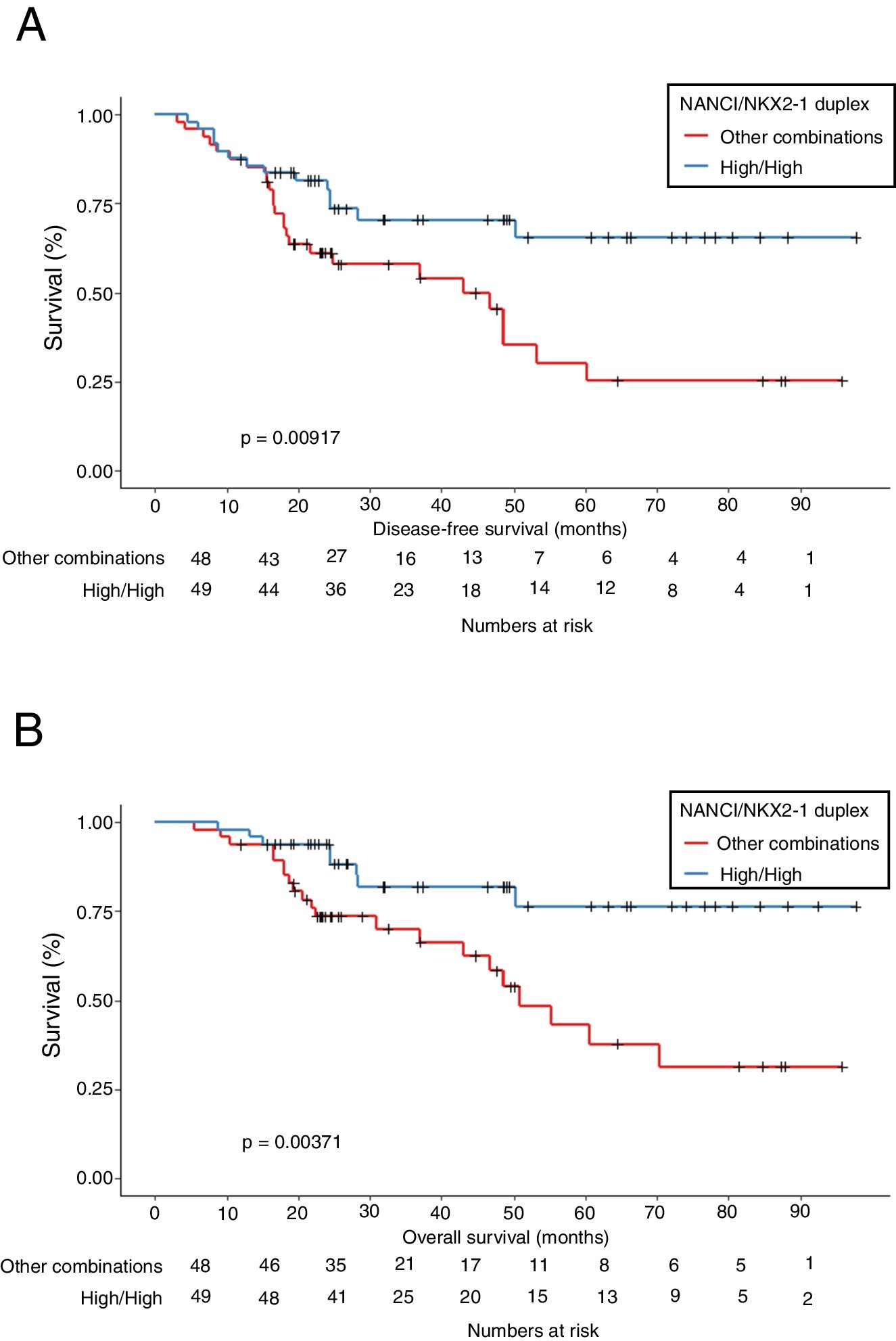
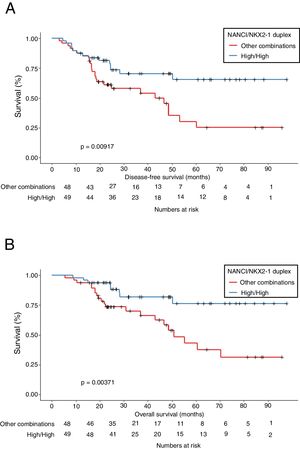
Mean OS was 81.4 months in patients with high NANCI/high NKX2-1, 41 months in those with high NANCI/low NKX2-1, 67.2 months in those with low NANCI/high NKX2-1, and 56.7 months in those with low NANCI/low NKX2-1 (P=0.024; Fig. 2D). Moreover, patients with high NANCI/high NKX2-1 duplex expression had longer DFS and OS than the other three groups (71.25 vs 46.3 months; P=0.009; 81.3 vs 56.1 months; P=0.004; Fig. 3A and B).
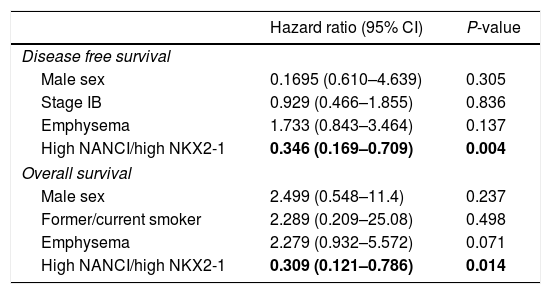
The multivariate analysis for DFS, including gender, stage, emphysema and NANCI/NKX2-1 duplex, also identified high NANCI/high NKX2-1 duplex expression as a factor for longer DFS (HR: 0.346, 95% CI, 0.169–0.709; P=0.004). In the multivariate analysis for OS, including gender, smoking status, emphysema and NANCI/NKX2-1 duplex, only high NANCI/high NKX2–1 duplex expression emerged as an independent factor for longer OS (HR: 0.309, 95% CI, 0.121–0.786; P=0.014; Table 2). All the analyses using the 8th TNM edition are shown in supplementary document 1.
Multivariate analysis for DFS and OS.
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Disease free survival | ||
| Male sex | 0.1695 (0.610–4.639) | 0.305 |
| Stage IB | 0.929 (0.466–1.855) | 0.836 |
| Emphysema | 1.733 (0.843–3.464) | 0.137 |
| High NANCI/high NKX2-1 | 0.346 (0.169–0.709) | 0.004 |
| Overall survival | ||
| Male sex | 2.499 (0.548–11.4) | 0.237 |
| Former/current smoker | 2.289 (0.209–25.08) | 0.498 |
| Emphysema | 2.279 (0.932–5.572) | 0.071 |
| High NANCI/high NKX2-1 | 0.309 (0.121–0.786) | 0.014 |
Bold values correspond to variables with p < 0.05.
Given the several shortfalls to the current approach for selecting surgical patients with a high risk of recurrence based on disease stage alone, the implementation of biomarkers for further stratification of risk has been studied in recent years. The prognostic role of single genes (TP53, BRCA, ERCC1, RRM1, HER2, KRAS), gene signatures, and immune biomarkers in NSCLC has been analyzed but the results require validation in prospective cohorts before their clinical application can be recommended.17 Despite these challenges, the discovery of newer molecular markers or markers that could elucidate NSCLC oncogenic functionality are currently a promising area of research.
In the present study, we have examined the expression of the lncRNA NANCI in human lung embryos and lung cancer and evaluated its potential as a prognostic biomarker in stage I NSCLC. During organogenesis, lung specification begins around day 28 of gestation and is regulated by NKX2-1 expression on the ventral side of the anterior foregut endoderm. Ventral evagination results in the formation of trachea and bronchial branching, a process where Wnt2/2b and beta-catenin signaling are necessary.8 In addition, BMP signaling promotes NKX2-1 respiratory endoderm specification via SOX2 repression.18 NANCI is a lncRNA located downstream from NKX2-1 that acts in cis to regulate NKX2-1. In turn, NKX2-1 directly inhibits NANCI expression, generating a negative feedback loop, indicating that NANCI/NKX2-1 duplex is essential for buffering NKX2-1 expression.14 In fact, our results reinforce this idea, since NANCI and NKX2-1 showed a high correlation in embryonic samples and in tumor tissues. Moreover, in the present study, we have shown that NANCI and NKX2-1 expression was present in late embryogenesis (E8), with increased levels during the pseudoglandular phase of human lung development (E8-E13) and the highest levels in adult normal tissue. Interestingly, in the NSCLC samples, NKX2-1 and NANCI expression were downregulated compared to normal lung tissue samples, sharing similar expression patterns to the early embryonic lung samples at weeks 8–10. This finding led us to speculate that diminished NKX2-1 and NANCI expression could be explained by dedifferentiation, reinforcing previous research that revealed significant similarities between early embryonic lung and lung cancer.9,19 There is growing evidence that several lncRNAs play a key role in maintaining homeostasis in embryogenesis.20 LncRNAs can activate or repress gene expression, acting as scaffolds to guide chromatin-modifying complexes21 and regulate neighboring protein-coding genes.22 The HOX family of genes,23 the lncRNA MALALT124 and the lncRNA Fendrr25 are also known to regulate both embryogenesis and carcinogenesis.
When we correlated NANCI levels with the main clinic–pathological characteristics, we observed that NANCI was upregulated in ADK patients compared to SCC. This correlation was also observed in NKX2-1 levels, which was in line with previous studies in lung cancer cohorts.12,26 However, not all clinic–pathological correlations found for NKX2-1 were also valid for NANCI levels. For example, NKX2-1 downregulation was associated with smoking,27 which had previously been studied functionally in thyroid gland development, where fetuses from smoke-exposed mothers had reduced NKX2-1 and GAT6 levels.28 Even though NANCI and NKX2-1 are strongly correlated, NANCI did not seem to be modified by smoke exposure. However, we found that patients with DLCO <60% had reduced expression of NANCI and NKX2-1. In emphysema, alveolar destruction is the pathophysiological cause of DLCO reduction.29 Abnormalities in DLCO can be found in smokers with early or mild emphysema.30
Finally, we evaluated the potential role of NANCI expression as a prognostic biomarker. Low NANCI levels were associated with shorter DFS (P=0.032) shorter OS (P=0.021) in stage I NSCLC patients. Moreover, confirming our previous results,12 low NKX2-1 levels were also associated with worse outcome. As NANCI downregulation alone does not adversely affect embryonic lung development and concurrent heterozygous mutations in both NANCI and NKX2-1 lead to persistent NKX2-1 deficiency,14 we analyzed the combinatory effect of both NKX2-1 and NANCI. Patients with high NANCI/high NKX2-1 expression showed the best prognosis, with longer DFS and longer OS, compared to patients with other combinations. In addition, high NANCI/high NKX2-1 expression emerged as the only independent prognostic factor for both DFS (HR: 0.346, P=0.004) and OS (HR: 0.309, P=0.014) in our cohort of stage I NSCLC patients, confirming its potential as prognostic biomarker.
To explore the prognostic impact of NANCI and NANCI/NKX2-1 according to the TNM 8th edition, we reclassified the cohort excluding 12 patients upstaged to IIA and confirmed the prognostic impact of NANCI in stage I NSCLC patients. In the multivariate analysis, the high/high NANCI/NKX2-1 combination remained an independent prognostic factor for OS (HR 0.29. P=0.012) (supplementary file 1).
To the best of our knowledge, this is the first study to report a prognostic impact for the lncRNA NANCI and for the duplex NANCI-NKX2-1 in stage I NSCLC. Although the exact mechanism of NANCI–NKX2-1 regulation in NSCLC is not fully addressed in the present study, our results indicate that NANCI and NKX2-1 expression may be important factors in the differentiation that occurs during the development from fetal to adult lung and that the assessment of NANCI/NKX2-1 levels in lung cancer can help to identify high-risk patients with stage I NSCLC.
ConclusionsNANCI/NKX2-1 duplex expression is correlated with prognosis in stage I NSCLC patients, defining subgroups of patients with differential outcomes who could benefit from a customized approach to treatment.
Data availabilityThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
FundingThis work was partially supported by grants from the Ministry of Economy, Industry and Competition, Agencia Estatal de Investigación co-financed with the European Union FEDER funds SAF2017-88606-P (AEI/FEDER, UE) and SDCSD from the Universitat de Barcelona. JJC and JC are APIF fellow of the Universitat de Barcelona. None of the funding bodies had any role in the design of the study and collection, analysis, and interpretation of data, or in writing the manuscript.
Authors’ contributionJM, AN, JC, JMa, JJC, BH performed the research; JM, AN, RM, MM designed and supervised the research; JM, AN analyzed the data, and wrote the manuscript; JM, NV, LM, JR, GF, RM selected cases and analyzed the clinical data; JR and GF provided samples and performed the histopathological review. All authors have seen and approved this final version of the manuscript and agree with the decision to submit.
Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
To CERCA programme/Generalitat de Catalunya.