Pulmonary arterial hypertension (PAH) is a rare disease characterized by cellular proliferation and vasoconstriction of the pulmonary arterial bed. PAH is a devastating disease with a high mortality despite available therapy.1 Three well-known pathways are involved in PAH pathophysiology (endothelin, nitric oxide, and prostacyclin), which are all therapeutic targets. The updated ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension recommend prostacyclin analogues in patients suffering from severe PAH, referring to WHO functional class (FC) III and IV.1 Prostacyclin is an endogenous prostanoid, that induces vasodilatation and inhibits platelet aggregation and cell proliferation, and patients with PAH present decreased prostacyclin production.2 Treprostinil, as prostacyclin analogue, is metabolically stable, long-acting, and can be administered subcutaneously using a portable small delivery system.3 Although subcutaneous administration implies less risk of systemic infection or catheter dislocation, subcutaneous infections and severe pain at infusion site are common and may make further treatment impossible in 7%–10% of the patients.3 Selexipag is a novel orally available non-prostacyclin selective prostacyclin receptor (IP receptor) agonist. Following absorption, it is hydrolyzed to an active more potent metabolite, which has a 7.9-h half-life, allowing for twice daily dosing. Common side effects (all drug class related) include headache, diarrhea, jaw pain, nausea, myalgia, vomiting, and flushings. Selexipag has shown efficacy in PAH, even in sequential triple combination,4 and it is currently approved for the treatment of PAH patients in WHO FC II-III, both in combination with endothelin receptor antagonist (ERA) and/or phosphodiesterase 5 inhibitors (iPDE-5) or in monotherapy.1 Given its oral administration, selexipag avoids the complications related to continuous parenteral administration. Hence, switching to selexipag maybe an attractive option in patients who were once started on a parental prostacyclin. However, little is known with regards to the aforementioned drug switch.5 Here, we present a successful case of transition from subcutaneous treprostinil to selexipag in a PAH patient reporting unbearable adverse events associated with treprostinil subcutaneous administration (Appendix A).
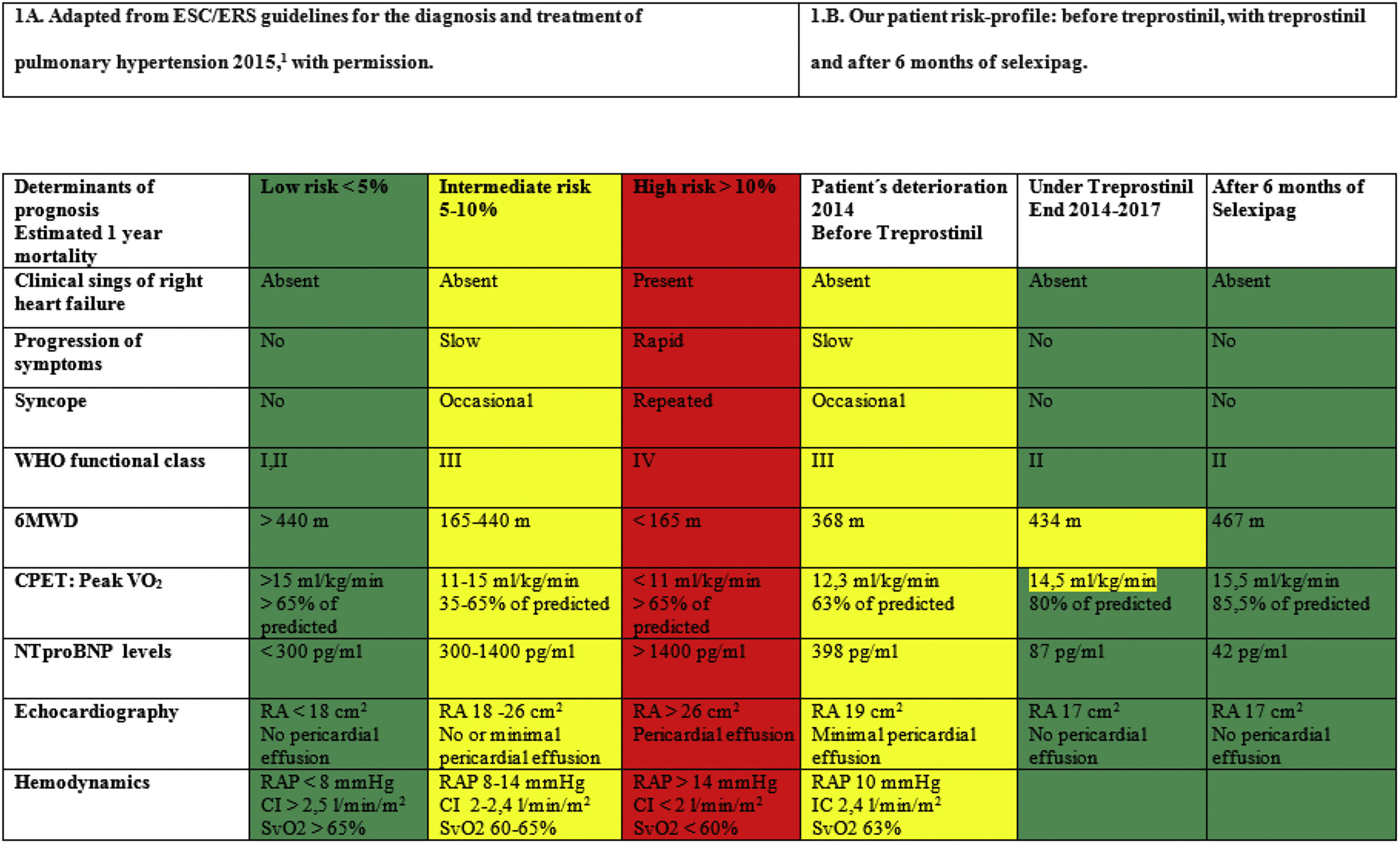
In August 2011 a 54-year-old woman was referred to our PAH Unit due to severe idiopathic PAH with a high-risk profile according to the prognosis assessment table suggested by current guidelines1 (Table 1A). After six months of combination therapy with the iPDE-5 sildenafil (80mg/8h) and the ERA ambrisentan (10mg/24h), the patient improved and achieved every low risk criteria (Table 1A). Later on, sildenafil was switched to the iPDE-5 tadalafil 40mg/24h due to nasal congestion. Following this, the patient remained stable in the low risk profile for two additional years. In early 2014, the patient deteriorated to the intermediate risk profile (Table 1A) and subcutaneous treprostinil was added and titrated up to 30ng/kg/min. With the triple regime (ERA + iPDE-5 + treprostinil), she quickly recovered her previous low-risk profile and remained stable for another 32 months. Unfortunately, our patient insistently complained about unbearable pain around treprostinil infusion site, and several subcutaneous infections were reported. Consequently, we planned progressive transition from subcutaneous treprostinil to oral selexipag after non-invasive risk profile assessment (Table 1B). Patient refused right heart catheterization. Progressive treprostinil down-titration and simultaneous selexipag up-titration was performed in outpatient clinic, under close supervision, throughout 8 weeks, so that every week, 4ng/kg/min of treprostinil were reduced and 200μg/12h were added. During the first 4 days of selexipag intake, patient reported mild diarrhoea, self-limited within the first week. No other side effects were reported. Patient ended up taking maximal doses of oral selexipag 1600μg/12h, plus her previous tadalafil and ambrisentan combination therapy unvaried. Six months after treprostinil withdrawal, the patient risk profile had slightly improved (Table 1B). Her functional capacity was better (both in 6min walking test distance and in cardiopulmonary exercise test) and quality of life drastically increased; not having to carry the infusion pump was a great relief.
Our case report suggests that transition from subcutaneous treprostinil to oral selexipag under strict clinical supervision may be a safe option for patients with controlled PAH, avoiding the undesirable side effects related to parenteral administration, meanwhile maintaining clinical stability. There is little experience with transition to oral selexipag from parenteral prostacyclin therapy,5 and our patient represents the first case of a subcutaneous treprostinil to oral selexipag switch. Further investigation is required to clarify whether the route of administration may influence efficacy and safety profile, and to help characterizing the patients that may benefit from this transition.
Finally, it should be noted that, we have relied on non-invasive prognostic markers (WHO FC, NTproBNP and 6MWD) for patient risk profile assessment since they have been validated recently in follow-up,6 added to patient's refusal to undergo another right heart catheterization. We added echocardiography and CPET in order to increase accuracy taking into account the lack of invasive data.
In conclusion, this case highlights how a safe transition from parenteral to oral prostacyclin pathway therapy in a stable PAH patient is possible. Thereby, avoiding the unbearable side effects associated with the parental route of prostacyclin administration.