The aim of this analysis was to describe the patterns of inhaled maintenance therapy according to risk level and to explore the determinants associated with the decision to prescribe inhaled corticosteroids (ICS) in addition to bronchodilator therapy according to risk level as strategy in the follow-up of COPD in daily clinical practice.
MethodsEPOCONSUL 2021 is a cross-sectional audit that evaluated the outpatient care provided to patients with a diagnosis of chronic obstructive pulmonary disease (COPD) in respiratory clinics in Spain with prospective recruitment between April 15, 2021 and January 31, 2022.
Results4225 patients from 45 hospitals in Spain were audited. Risk levels were analyzed in 2678 patients. 74.5% of patients were classified as high risk and 25.5% as low risk according to GesEPOC criteria. Factors associated with the prescription of ICS in low-risk COPD were symptoms suggestive of asthma [OR: 6.70 (3.14–14.29), p<0.001], peripheral blood eosinophilia>300mm3 [OR: 2.16 (1.10–4.24), p=0.025], and having a predicted FEV1%<80% [OR: 2.17 (1.15–4.08), p=0.016]. In high-risk COPD, factors associated with triple therapy versus dual bronchodilator therapy were a mMRC dyspnea score of ≥2 [OR: 1.97 (1.41–2.75), p<0.001], symptoms suggestive of asthma [OR: 6.70 (3.14–14.29), p<0.001], and a predicted FEV1%<50% [OR: 3.09 (1.29–7.41), p<0.011].
ConclusionsInhaled therapy in the follow-up of COPD does not always conform to the current guidelines. Few changes in inhaled therapy are made at follow-up visits. The use of ICS is common in COPD patients who meet low-risk criteria in their follow-up and triple therapy in high-risk COPD patients is used as an escalation strategy in patients with high clinical impact. However, a history of exacerbations and eosinophil count in peripheral blood were not factors predicting triple therapy.
The management of stable chronic obstructive pulmonary disease (COPD) primarily aims to improve symptoms and quality of life, optimize lung function, reduce exacerbations, and improve exercise tolerance. Updates to the clinical practice guidelines for the management of COPD provide pharmacological treatment algorithms based on symptoms and exacerbation history.1–3
The Spanish National Guidelines for COPD care (GesEPOC) establish risk stratification of COPD patients as a key step in planning therapeutic interventions and deciding on inhaled pharmacological treatment.4–7 At the lowest risk level, the prescription of bronchodilators is recommended, and anti-inflammatory treatment is not indicated. In patients with a higher level of risk, the need for therapeutic interventions will be greater. Therefore, the use of inhaled triple therapy consisting of a long-acting β2-agonist (LABA), a long-acting muscarinic antagonist (LAMA) and an inhaled corticosteroid (ICS) has been recommended for years as part of treatment escalation strategies in COPD and, more particularly, in patients who still have clinically significant symptoms that adversely impact their quality of life or those who have a higher risk of exacerbation despite receiving dual therapy.8–11 However, in daily clinical practice, the prescription of triple inhaled therapy in high-risk patients, i.e., adding an ICS to the LABA/LAMA combination, is still a controversial issue. Current evidence suggests that the benefit of adding an ICS to bronchodilator therapy is associated with certain clinical conditions, the most important of which being blood eosinophil concentration, frequency and severity of exacerbations, and active smoking.12,13
Different studies indicate that there is an overprescription of ICS and a use that does not fit the different patient types.14–16 However, most of these studies are conducted in automated databases or in primary care, which does not allow understanding the determinants of pharmacological prescription in daily clinical practice in COPD follow-up in secondary care. Consequently, there is a need to assess what the key elements are that pulmonologists base their therapeutic decisions on in daily clinical practice in the adjustment of maintenance therapy in COPD follow-up.
This study is an analysis of the EPOCONSUL 2021 clinical audit that evaluated the outpatient care provided to patients with COPD in respiratory clinics in Spain. We aimed to describe the maintenance inhaled therapy performed by the patient in follow-up by pulmonology according to risk level and to identify the determinants associated with the use an ICS in addition to bronchodilator therapy in low risk and the use of triple therapy versus dual bronchodilator therapy as a strategy for adequacy of maintenance treatment in high risk.
MethodologyThe methodology of the EPOCONSUL audit has been previously reported.17,18 Briefly, the EPOCONSUL audit promoted by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) was designed to evaluate outpatient care provided to patients with COPD in respiratory clinics in Spain as an observational non-interventional cross-sectional study.
The Spanish Society of Pneumology and Thoracic Surgery sent an official invitation to participate in the study to all the respiratory units in Spain with outpatient respiratory clinics according to the Ministry of Health registry and the SEPAR member registry. Recruitment was intermittent; every month, each investigator recruited the clinical records of the first 10 patients identified as being diagnosed with COPD that were seen in the outpatient respiratory clinic. Subsequently, the patients identified were reevaluated to determine if they met the inclusion/exclusion criteria described in Appendix 1. The information collected was historical in nature for the clinical data from the last visit pre-pandemic (conducted before March 2020) and previous visits, and the information about hospital resources was concurrent and is described in Appendix 2. The level of risk was defined according to GesEPOC criteria (post-bronchodilator FEV1%, degree of dyspnea and history of exacerbations) described in Appendix 3. COPD control was evaluated according to GesEPOC criteria based on two components: impact and stability.2,19,20 These criteria are described in Appendix 4.
The protocol was approved by the Ethics Committee of the Hospital Clínico San Carlos (Madrid, Spain; internal code 20/722-E). Additionally, according to current research laws in Spain, the ethics committee at each participating hospital evaluated and agreed to the study protocol. The need for informed consent was waived because ours is a clinical audit, in addition to the non-interventional nature of the study, the anonymization of data and the need to blindly evaluate clinical performance. To avoid modifications to the usual clinical practice and preserve the blinding of the clinical performance evaluation, the medical staff responsible for the outpatient respiratory clinic was not informed about the audit.
Statistical analysisQualitative variables were summarized by their frequency distribution and quantitative variables by their median and interquartile range.
Univariate and multivariate analysis to study the factors associated with the prescription of an ICS in the low-risk level and with the prescription of triple therapy in the high-risk level were analyzed via a multilevel logistic regression model. Each multilevel analysis included two levels: the individual or patient level (level 1) and the hospital level (level 2). Candidate adjustment variables (patient characteristics and hospital resources) for both multivariate models were those that presented a p value<0.05 in the univariate analysis and/or were clinically relevant. Statistical significance was assumed as p<0.05. All analyses were performed using Stata software version 17 (StataCorp LLC, College Station, TX, USA).
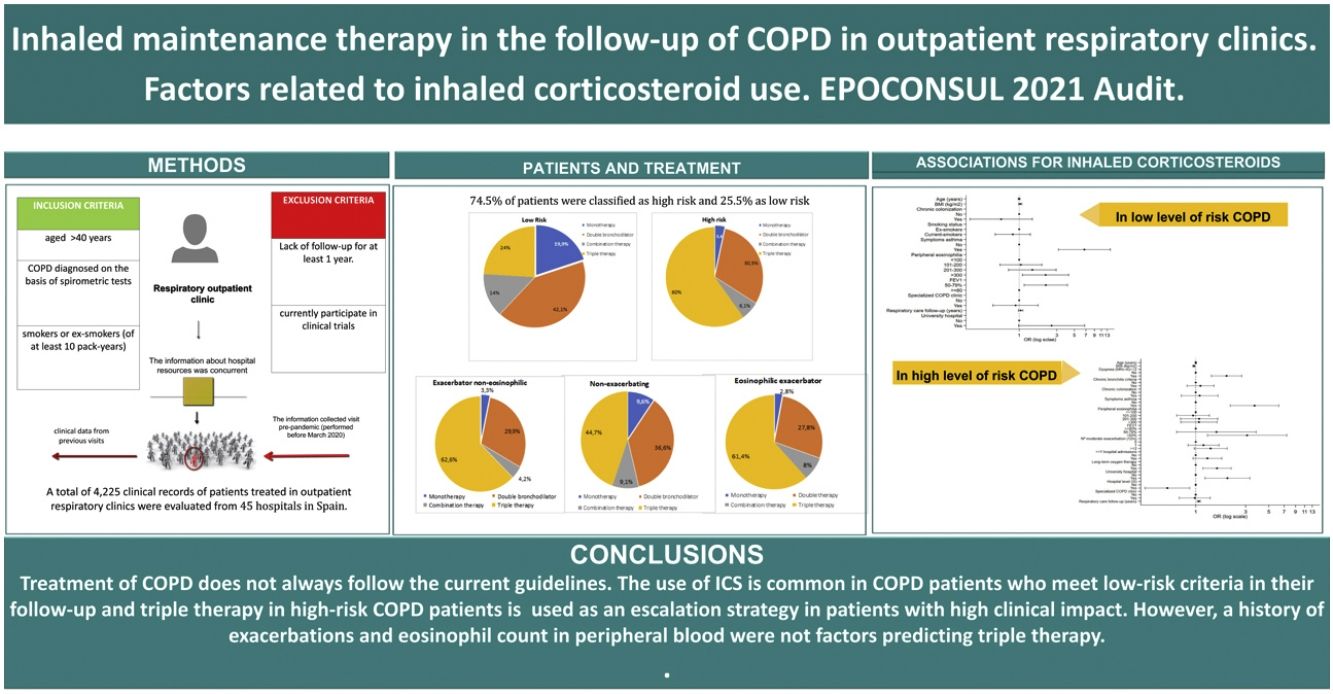
ResultsPopulationA total of 4225 patients with a diagnosis of COPD from 45 centers were audited. Of them, a total of 2678 patients on inhaled therapy and meeting all GesEPOC criteria for defining risk levels were analyzed. The sampling process is shown in Fig. 1 and in Appendix 5 the comparison of patients excluded and included in this analysis. Among the total patient population evaluated by risk level, 1994 (74.5%) patients were classified as high risk and 684 (25.5%) as low risk. Table 1 shows the distribution of the criteria that defined patients as high risk. In total, 15.4% of high-risk patients met 3 criteria, and 29.3% met a single criterion. The most common high-risk criterion was a mMRC dyspnea score of ≥2, and this was detected in 76.1% of the high-risk patients compared to the criterion of frequent exacerbations, which was detected in 43% of high-risk patients. Demographic and clinical characteristics by risk level are shown in Table 2.
Distribution of the patients according to the criteria that define risk level.
| All patients (n=2678) | |
|---|---|
| Low risk level, n (%) | 684 (25.5) |
| High risk level, n (%) | 1994 (74.5) |
| It has only one high risk criterion, n (%) | 785 (29.3) |
| Only degree of dyspnea≥2 (MRC-m) | 395 (14.7) |
| Only FEV1<50% predicted | 260 (9.7%) |
| Only ≥2 moderate exacerbations and/or ≥1 graves exacerbations | 130 (4.9%) |
| It has two high-risk criteria, n (%) | 797 (29.7) |
| Degree of dyspnea≥2 (MRC-m) and FEV1<50% predicted | 478 (17.8) |
| Degree of dyspnea≥2 (MRC-m) and [≥2 moderate exacerbations and/or ≥1 graves exacerbations] | 233 (8.7) |
| FEV1<50% predicted and [≥2 moderate exacerbations and/or ≥1 graves exacerbations] | 86 (3.2) |
| It has three high-risk criteria, n (%) | 412 (15.4) |
Data are represented as percentages; FEV1%: post-bronchodilator FEV1 percent predicted.
Characteristics and resources in care of the total patients evaluated by risk level.
| All patients with assessed COPD risk level (n=2678) | Patients low-risk (n=684) | Patients high-risk (n=1994) | p | |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Gender (male), n (%) | 1911 (71.4) | 452 (66.1) | 1459 (73.2) | <0.001 |
| Age (years), m (SD) | 69.7 (9) | 68.3 (9.0) | 70.2 (9.0) | <0.001 |
| ≤55, n (%) | 170 (6.4) | 62 (9.1) | 108 (5.4) | <0.001 |
| 56–69, n (%) | 1129 (42.2) | 310 (45.3) | 819 (41.1) | |
| ≥70, n (%) | 1378 (51.5) | 312 (45.6) | 1066 (53.5) | |
| Smoking status | ||||
| Current smokers, n (%) | 677 (25.3) | 213 (31.1) | 464 (23.3) | <0.001 |
| Ex-smokers, n (%) | 2001 (74.7) | 471 (68.9) | 1530 (76.7) | |
| Pack-years, m (SD) | 49.6 (23.7) | 44.5 (22.9) | 51.3 (23.7) | <0.001 |
| BMI kg/m2, m (SD) | 27.6 (5.5) | 27.5 (4.6) | 27.6 (5.8) | 0.671 |
| ≤21, n (%) | 237 (8.8) | 40 (6.1) | 197 (10.4) | 0.001 |
| ≥30, n (%) | 1724 (67.8) | 475 (72.4) | 1249 (66.2) | 0.001 |
| Comorbidity | ||||
| Charlson index, median, IQR | 1 (1–3) | 1 (1–2) | 2 (1–3) | <0.001 |
| Charlson index≥3, n (%) | 740 (27.7) | 145 (21.3) | 595 (29.9) | <0.001 |
| History of asthma, n (%) | 160 (6.3) | 50 (7.8) | 110 (5.7) | 0.056 |
| Dyspnea (MRC-m)≥2, n (%) | 1518 (56.7) | 0 | 1518 (76.1) | <0.001 |
| CAT questionnaire>10, n (%) | 743 (67.6) | 96 (40.7) | 647 (75) | <0.001 |
| Chronic bronchitis criteria, n (%) | 968 (36.1) | 189 (27.6) | 779 (39.1) | <0.001 |
| Chronic colonization, n (%) | 347 (13) | 59 (8.6) | 288 (14.4) | <0.001 |
| Symptoms suggestive of asthma, n (%) | 287 (10.7) | 71 (10.4) | 216 (10.8) | 0.741 |
| Peripheral eosinophilia, median (IQR) | 200 (100–300) | 200 (100–300) | 200 (100–300) | 0.933 |
| ≤100mm3, n (%) | 483 (26.9) | 115 (25.5) | 368 (27.4) | 0.626 |
| 101–200mm3, n (%) | 542 (30.2) | 147 (32.6) | 395 (29.4) | |
| 201–300mm3, n (%) | 356 (19.8) | 87 (19.3) | 269 (20) | |
| >300mm3, n (%) | 415 (23.1) | 102 (22.6) | 313 (23.3) | |
| FEV1 (%), m, (SD) | 52.3 (18.3) | 68.6 (12.8) | 46.8 (16.5) | <0.001 |
| <50%, n (%) | 1236 (46.2) | 0 | 1236 (62) | <0.001 |
| 50–79%, n (%) | 1244 (46.5) | 560 (81.9) | 684 (34.3) | |
| ≥80%, n (%) | 198 (7.4) | 124 (18.1) | 74 (3.7) | |
| Number of moderate exacerbations in the last year, median, IQR | 0 (0–1) | 0 (0–1) | 1 (0–1) | <0.001 |
| % patients with moderate exacerbations, n (%) | ||||
| 0 exacerbations | 1480 (55.3) | 500 (73.1) | 980 (49.1) | <0.001 |
| 1 exacerbation | 713 (26.6) | 184 (26.9) | 529 (26.5) | |
| ≥2 moderate exacerbations in the last year | 485 (18.1) | 0 | 485 (18.1) | |
| Number of graves exacerbations in the last year, median, IQR | 0 (0–0) | 0 (0–0) | 0 (0–1) | <0.001 |
| % ≥1 hospital admissions in the last year, n (%) | 550 (20.5) | 0 | 550 (27.6) | <0.001 |
| BODE, m, (SD) | 3.9 (2.1) | 1.1 (1.2) | 4.3 (1.9) | <0.001 |
| GesEPOC phenotype, n (%) | ||||
| Non-exacerbating | 1817 (75.1) | 684 (100) | 1133 (65.3) | <0.001 |
| Exacerbator non-eosinophilic | 425 (17.6) | 0 | 425 (17.6) | |
| Eosinophilic exacerbator | 176 (7.3) | 0 | 176 (7.3) | |
| Clinical control level according to GesEPOC, n (%) | ||||
| Good clinical control | 880 (50.4) | 419 (89) | 461 (36.2) | <0.001 |
| Poor control | 865 (49.6) | 52 (11) | 813 (63.8) | |
| Roflumilast | 77 (2.9) | 4 (0.6) | 73 (3.7) | <0.001 |
| Mucolitics | 120 (4.5) | 9 (1.3) | 111 (5.6) | <0.001 |
| Methylxanthines | 45 (1.7) | 1 (0.1) | 44 (2.2) | <0.001 |
| Antibiotics | 149 (5.6) | 10 (1.5) | 139 (7) | <0.001 |
| Long-term oxygen therapy, n (%) | 661 (24.7) | 20 (2.9) | 641 (32.1) | <0.001 |
| Home ventilation, n (%) | 213 (8) | 27 (3.9) | 186 (9.3) | <0.001 |
| Respiratory rehabilitation, n (%) | 371 (13.9) | 36 (5.3) | 335 (16.8) | <0.001 |
| Resources in care | ||||
| Attended in specialized COPD outpatient clinic, n (%) | 1187 (44.5) | 200 (29.2) | 987 (49.7) | <0.001 |
| Respiratory care follow-up (years) median, IQR | 5.7 (3.5–8.7) | 4.7 (3.1–7.3) | 6 (3.7–9.1) | <0.001 |
| Level of complexity of hospital, n (%) | ||||
| Secondary | 529 (19.8) | 182 (26.6) | 347 (17.4) | <0.001 |
| Tertiary | 2149 (80.29 | 502 (73.4) | 1647 (82.6) | |
| University hospital, n (%) | 2257 (84.3) | 570 (83.3) | 1687 (84.6) | 0.431 |
| Availability of specialized COPD outpatient clinic, n (%) | 1767 (66) | 380 (55.6) | 1387 (69.6) | <0.001 |
| Availability of nursing consultation, n (%) | 1532 (57.2) | 378 (55.3) | 1154 (57.9) | 0.234 |
Data are represented as mean (standard deviation) or absolute (relative) frequencies or median (IQR: interquartile range); BMI: body mass index; FEV1%: post-bronchodilator FEV1 percent predicted; CAT: COPD assessment test; BODE: body mass index, airflow obstruction, dyspnea, and exercise capacity; GesEPOC: Spanish National Guideline for COPD.
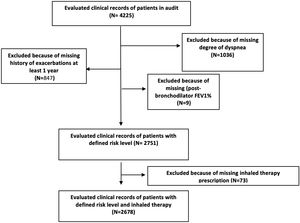
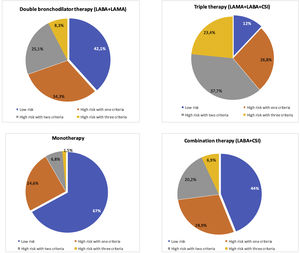
Fig. 2 shows the distribution of COPD inhaled treatments according to risk level and clinical phenotype. The most commonly used option in low-risk patients was combinations of long-acting bronchodilators or bronchodilator dual therapy (42.1%) while it was triple therapy (60%) in high-risk patients. Triple therapy was the most commonly prescribed option in all clinical phenotypes (44.7% in non-exacerbators and 61.4% in eosinophilic exacerbators). Fig. 3 shows the positioning of the different inhaled therapy strategies.
Distribution of inhaled therapy according to COPD risk level and phenotype GesEPOC. Footnote: Data are represented as percentages; LABA: long-acting beta-2 agonists; LAMA: long-acting antimuscarinic agents; CSI: inhaled corticosteroids; monotherapy (LAMA or LABA), double bronchodilator therapy (LAMA+LABA); combination therapy (LABA+CSI); triple therapy: LAMA+LABA+CSI.
Distribution of inhaled therapy according to COPD risk level criteria. Footnote: Data are represented as percentages; LABA: long-acting beta-2 agonists; LAMA: long-acting antimuscarinic agents; CSI: inhaled corticosteroids; monotherapy (LAMA or LABA), double bronchodilator therapy (LAMA+LABA); combination therapy (LABA+CSI); triple therapy: LAMA+LABA+CSI.
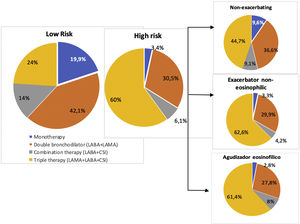
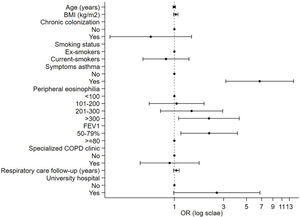
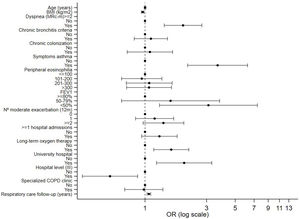
In Tables 3 and 4, factors associated with the prescription of an ICS in patients with COPD according to the level of risk are presented. In patients with a low level of risk, symptoms suggestive of asthma or asthma comorbidity had the highest association with ICS prescription [OR: 6.70 (3.14–14.29), p<0.001], followed by peripheral eosinophilia>300mm3 [OR: 2.16 (1.10–4.24), p=0.025]. In addition, those with a predicted FEV1%<80% had a higher probability of being prescribed an ICS [OR: 2.17 (1.15–4.08), p=0.016]. The multivariate analysis of patients with a high level of risk receiving triple therapy versus dual bronchodilator therapy showed that patients with a mMRC dyspnea score of ≥2 had 1.9 times a higher probability of receiving triple therapy. Furthermore, those with symptoms suggestive of asthma had a 3.3 times higher probability of receiving maintenance triple therapy. In addition, those with a predicted FEV1%<50% had a higher probability of being prescribed triple therapy [3.09 (1.29–7.41), p<0.011].
Factors associated with the prescription of ICS in patients with a low level of risk.
| No prescription ICS (n=424) | Prescription ICS (n=260) | Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p | |
|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | ||||||
| Age (years), m (SD) | 67.7 (8.9) | 69.2 (8.9) | 1.02 (0.99–1.03) | 0.067 | 0.99 (0.97–1.02) | 0.863 |
| Gender, male, n (%) (yes) | 283 (66.7) | 169 (65) | 0.87 (0.60–1.25) | 0.457 | ||
| Tobacco | ||||||
| Pack-years, m (SD) | 44.2 (22.4) | 45 (23.7) | ||||
| Ex-smokers, n (%) | 276 (65.1) | 195 (75) | 1 | 0.007 | 1 | 0.475 |
| Current smokers, n (%) | 148 (34.9) | 65 (25) | 0.59 (0.40–0.86) | 0.82 (0.49–1.38) | ||
| BMI kg/m2, m (SD) | 27.2 (4.5) | 27.9 (4.6) | 1.02 (0.98–1.05) | 0.267 | 1.03 (0.98–1.08) | 0.203 |
| Comorbidity | ||||||
| Charlson index, median, IQR | 1 (1–2) | 1 (1–2) | 1.03 (0.93–1.14) | 0.503 | ||
| Charlson index≥3, n (%) (yes) | 84 (19.9) | 61 (23.6) | 1.11 (0.73–1.67) | 0.625 | ||
| Chronic bronchitis criteria, n (%) (yes) | 116 (27.4) | 73 (28.1) | 1.24 (0.81–1.90) | 0.323 | ||
| Chronic colonization, n % (yes) | 44 (10.4) | 15 (5.8) | 0.65 (0.29–1.47) | 0.306 | 0.59 (0.23–1.46) | 0.257 |
| Symptoms suggestive of asthma or comorbidity of asthma, n (%), (yes) | 16 (3.8) | 55 (21.2) | 6.28 (3.34–11.8) | <0.001 | 6.70 (3.14–14.29) | <0.001 |
| Peripheral eosinophilia, median (IQR) | 167 (100–300) | 210 (140–360) | 1.0 (1.00–1.02) | 0.004 | ||
| ≤100mm3, n (%) | 77 (28.4) | 38 (21.1) | 1 | 1 | ||
| 101–200mm3, n (%) | 100 (36.9) | 47 (26.1) | 0.92 (0.52–1.62) | 0.775 | 1.05 (0.57–1.94) | 0.871 |
| 201–300mm3, n (%) | 46 (17) | 41 (22.8) | 1.51 (0.79–2.86) | 0.211 | 1.46 (0.73–2.94) | 0.278 |
| >300mm3, n (%) | 48 (17.7) | 54 (30) | 2.17 (1.16–4.04) | 0.015 | 2.16 (1.10–4.24) | 0.025 |
| Severity FEV1 (%), m, (SD) | 69.7 (13.2) | 66.8 (11.9) | 0.97 (0.96–0.99) | <0.00 | ||
| ≥80%, n (%) | 87 (20.5) | 37 (14.2) | 1 | 1 | 1 | |
| 50–79%, n (%) | 337 (79.5) | 223 (85.8) | 1.92 (1.20–3.06) | 0.006 | 2.17 (1.15–4.08) | 0.016 |
| % of patients with moderate exacerbation | ||||||
| 0 exacerbation, n (%) | 303 (71.5) | 197 (75.8) | 1 | |||
| 1 exacerbation, n (%) | 121 (28.5) | 63 (24.2) | 1.23 (0.80–1.89) | 0.335 | ||
| BODE index, m (SD) | 1.1 (1.2) | 1.2 (1.2) | 1.08 (0.79–1.49) | 0.602 | ||
| Clinical control level according to GesEPOC | ||||||
| Good clinical control, n (%) | 276 (89) | 143 (88.8) | 1 | |||
| Poor clinical control, n (%) | 34 (11) | 18 (11.2) | 1.56 (0.72–3.37) | 0.251 | ||
| Long-term oxygen therapy, n (%), (yes) | 9 (2.1) | 11 (4.2) | 2.08 (0.79–5.48) | 0.136 | ||
| Resources in care | ||||||
| Attended in specialized COPD outpatient clinic, n (%), (yes) | 112 (26.4) | 88 (33.8) | 1.65 (1.00–2.73) | 0.051 | 0.89 (0.46–1.73) | 0.754 |
| Respiratory care follow-up (years) median, IQR | 4.3 (3–6.4) | 5.7 (3.3–8.5) | 1.08 (1.03–1.13) | <0.001 | 1.04 (0.97–1.11) | 0.182 |
| University hospital, n (%), (yes) | 340 (80.2) | 230 (88.5) | 2.68 (1.02–7.00) | 0.046 | 2.57 (0.98–6.74) | 0.054 |
| Level of hospital III, n (%), (yes) | 311 (73.3) | 191 (75.3) | 1.10 (0.50–2.45) | 0.807 | ||
| Availability of specialized COPD outpatient clinic, n (%), (yes) | 243 (57.3) | 137 (52.7) | 0.81 (0.40–1.64) | 0.561 | ||
| Availability of nursing consultation, n (%), (yes) | 233 (55) | 145 (55.8) | 1.18 (0.56–2.25) | 0.755 | ||
Data are represented as mean (standard deviation) or absolute (relative) frequencies or median (IQR: interquartile range); BMI: body mass index; FEV1%: post-bronchodilator FEV1 percent predicted; BODE: body mass index, airflow obstruction, dyspnea, and exercise capacity; GesEPOC: Spanish National Guideline for COPD.
Factors associated with inhaled triple therapy treatment in high-risk COPD.
| N 1805 | Double bronchodilator therapy (n=609) | Triple therapy (n=1196) | OR (95% CI) unadjusted | p value | OR (95%CI) adjusted | p |
|---|---|---|---|---|---|---|
| Demographic and clinical characteristics | ||||||
| Age (years), m (SD) | 69.7 (8.7) | 70.4 (9.1) | 1.01 (1.00–1.02) | 0.047 | 1.00 (0.98–1.01) | 0.851 |
| Gender, male, n (%), (yes) | 450 (74) | 871 (72.8) | 0.95 (0.75–1.20) | 0.689 | ||
| BMI kg/m2, m (SD) | 28.3 (6.2) | 27.1 (5.5) | 0.96 (0.94–0.98) | <0.001 | 0.95 (0.93–0.98) | <0.001 |
| Tobacco | ||||||
| Pack-years, m (SD) | 50.9 (23.6) | 52 (23.8) | ||||
| Ex-smokers, n (%) | 463 (76) | 924 (77.3) | 1 | 0.508 | ||
| Current smokers, n (%) | 146 (24) | 272 (22.7) | 0.92 (0.72–1.17) | |||
| Dyspnea (mMRC)≥2, n (%), (yes) | 433 (71.1) | 960 (80.3) | 1.78 (1.39–2.28) | <0.001 | 1.97 (1.41–2.75) | <0.001 |
| Chronic bronchitis criteria, n (%), (yes) | 212 (34.8) | 512 (42.8) | 1.51 (1.21–1.89) | <0.001 | 1.11 (0.82–1.49) | 0.489 |
| Chronic colonization, n (%), (yes) | 72 (11.8) | 198 (16.6) | 1.66 (1.20–2.31) | 0.002 | 1.09 (0.72–1.63) | 0.673 |
| Symptoms suggestive of asthma, n (%), (yes) | 29 (4.8) | 162 (13.5) | 3.05 (1.98–4.72) | <0.001 | 3.65 (2.13–6.22) | <0.001 |
| Peripheral eosinophilia, median (IQR) | 200 (100–300) | 200 (100–300) | 1.00 (0.99–1.00) | 0.386 | ||
| ≤100mm3, n (%) | 111 (27.5) | 231 (28.2) | 1 | 1 | ||
| 101–200mm3, n (%) | 125 (30.9) | 237 (29%) | 0.94 (0.66–1.30) | 0.673 | 1.94 (0.65–1.35) | 0.752 |
| 201–300mm3, n (%) | 80 (19.8) | 158 (19.3) | 1.01 (0.69–1.48) | 0.941 | 1.07 (0.71–1.62) | 0.718 |
| >300mm3, n (%) | 88 (21.8) | 192 (23.5) | 1.11 (0.76–1.62) | 0.564 | 1.07 (0.71–1.60) | 0.725 |
| FEV1 (%), m, (SD) | 51 (15.6) | 43. (15.5) | 0.96 (0.96–0.97) | <0.001 | ||
| ≥80, n (%) | 28 (4.6) | 25 (2.1) | 1 | 1 | ||
| 50–79%, n (%) | 261 (42.9) | 332 (27.8) | 1.44 (0.80–2.58) | <0.001 | 1.57 (0.65–1.35) | 0.307 |
| <50%, n (%) | 320 (52.5) | 839 (70.2) | 2.92 (1.64–5.22) | <0.001 | 3.09 (1.29–7.41) | 0.011 |
| N° moderate exacerbations in the last year, median, (IQR) | 0 (0–1) | 1 (0–2) | 1.39 (1.24–1.55) | |||
| % of patients with moderate exacerbation | ||||||
| 0 exacerbation, n (%) | 337 (55.3) | 552 (46.2) | 1 | 0.028 | 1 | 0.323 |
| 1 exacerbation, n (%) | 155 (25.5) | 321 (26.8) | 1.32 (1.03–1.70) | <0.001 | 1.18 (0.84–1.66) | 0.078 |
| ≥2 moderate exacerbations in the last year, n (%) | 117 (19.2) | 323 (27) | 2.05 (1.54–2.73) | 1.38 (0.96–1.99) | ||
| % ≥1 hospital admissions in the last year, (%), (yes) | 150 (24.6) | 358 (29.9) | 1.48 (1.16–1.90) | 0.001 | 1.29 (0.94–1.77) | 0.111 |
| BODE index, m (SD) | 3.6 (1.8) | 4.6 (1.8) | 1.45 (1.27–1.67) | <0.001 | ||
| GesEPOC phenotype, n (%) | ||||||
| Non exacerbator | 377 (68.2) | 648 (63.4) | 1 | |||
| Non eosinophilic exacerbator | 127 (23) | 266 (26) | 1.36 (1.03–1.80) | 0.027 | ||
| Eosinophilic exacerbator | 49 (8.9) | 108 (10.6) | 1.29 (0.88–1.88) | 0.184 | ||
| Clinical control level according to GesEPOC, n (%) | ||||||
| Good clinical control | 164 (43.5) | 253 (31.8) | 1 | |||
| Poor clinical control | 213 (56.5) | 542 (68.2) | 1.88 (1.42–2.48) | <0.001 | ||
| Long-term oxygen therapy, n (%), (yes) | 137 (22.5) | 474 (39.6) | 2.36 (1.86–2.98) | <0.001 | 1.59 (1.16–.18) | 0.003 |
| Resources in care | ||||||
| University hospital n (%), (yes) | 478 (78.5) | 1047 (87.5) | 2.09 (1.22–3.55) | 0.006 | 2.01 (1.22–3.29) | 0.006 |
| Level of hospital III, n (%), (yes) | 521 (85.6) | 961 (80.4) | 0.69 (0.42–1.12) | 0.138 | 0.53 (0.32–0.87) | 0.013 |
| Attended in specialized COPD outpatient clinic, n %, (yes) | 269 (44.4) | 630 (52.9) | 1.46 (1.12–1.89) | 0.004 | 0.97 (0.69–1.37) | 0.903 |
| Respiratory care follow-up (years), median (IQR) | 5.4 (3.4–8.1) | 6.6 (4–9.5) | 1.07 (1.04–1.09) | <0.001 | 1.07 (1.03–1.10) | <0.001 |
| Availability of nursing consultation, n %, (yes) | 371 (60.9) | 720 (60.2) | 0.87 (0.58–1.32) | |||
Data are represented as mean (standard deviation) or absolute (relative) frequencies or median (IQR: interquartile range); BMI: body mass index; FEV1%: post-bronchodilator FEV1 percent predicted; BODE: body mass index, airflow obstruction, dyspnea, and exercise capacity; GesEPOC: Spanish National Guideline for COPD.
In Figs. 4 and 5, overviews of the main associations separately by ICS prescription in low-risk COPD and inhaled triple therapy prescription in high-risk COPD.
Change in inhaled therapy prescribedTable 5 shows the distribution of change in inhaled therapy according to risk level and COPD control. In 453 cases (25.9%) did not modify their inhaled therapy prescription in the last visit. The most frequent change made was to a similar regimen.
Change in prescribed inhaled therapy.
| All | Good control (n=880) | p | Poor control (n=865) | p | |||
|---|---|---|---|---|---|---|---|
| 1745 | Patients low-risk (n=419) | Patients high-risk (n=461) | Patients low-risk (n=52) | Patients high-risk (n=813) | |||
| Any change in prescribed inhaled therapy is carried out, n (%) | 453 (25.9) | 84 (20) | 94 (20.4) | 0.899 | 12 (23.1) | 263 (32.3) | 0.164 |
| Change performed, n (%) | |||||||
| Scaling (increased or added) | 172 (38) | 23 (27.4) | 36 (38.3) | 0.303 | 1 (8.3) | 112 (42.6) | 0.055 |
| De-escalate (decrease or remove) | 52 (11.5) | 15 (17.9) | 14 (14.9) | 2 (16.7) | 21 (8) | ||
| Changes to similar regime | 229 (50.5) | 46 (54.8) | 44 (46.8) | 9 (75) | 130 (49.4) | ||
| Referred reason for the change, n (%) | 0.641 | 0.003 | |||||
| By level of control | 223 (59.6) | 34 (50) | 43 (60.6) | 2 (20) | 144 (64) | ||
| By undesired effects | 37 (9.9) | 5 (7.4) | 5 (7) | 2 (20) | 25 (11.1) | ||
| By compliance | 48 (12.8) | 10 (14.7) | 8 (11.3) | 1 (10) | 29 (12.9) | ||
| By inhalation technique | 66 (17.7) | 19 (27.9) | 15 (21.1) | 5 (50) | 27 (12) | ||
The aim of this study was to evaluate the maintenance inhaled therapy of COPD patients under follow-up in pulmonology outpatients using real data generated in a clinical audit performed in Spain. This analysis describes pharmacological prescription patterns according to COPD risk level and explores the determinants associated with the decision to prescribe an ICS in addition to bronchodilator treatment in low risk and the use of triple therapy versus dual bronchodilator therapy as a strategy for adequacy of maintenance treatment in high risk.
Our key takeaways are that we have observed that the most common inhaled therapeutic strategy in low-risk patients was dual bronchodilator therapy (42.1%), although it is worth noting that the use of ICS in addition to bronchodilator therapy as a maintenance treatment for low-risk COPD remains present in more than one third of patients, with factors associated with the prescription of ICS identified as being symptoms suggestive of asthma, peripheral eosinophilia >300mm3 and moderate airflow obstruction.
Triple therapy continues to be the most frequently used regimen in high-risk patients (60%), being prescribed in twice as many patients as dual bronchodilator therapy, with factors associated with triple therapy being identified as having symptoms suggestive of asthma, dyspnea ≥2 and severe airflow obstruction. However, a history of exacerbations, eosinophil count in peripheral blood and smoking were not maintained as factors predicting triple therapy versus dual bronchodilator therapy in high-risk patients.
The results show a frequent use of ICS in patients with low-risk COPD under follow-up. Our study showed that approximately one third of patients with low-risk COPD received LABA+CSI combination therapy or triple therapy, a range that matches that observed in previous studies from over 5 years ago in Spain21,22 (27.5%), Japan23 (21%), France24 (33%), the United Kingdom25 (32%) and Greece26 (44.8%). Overall, this indicates that the prescription practices observed in Spain do not differ from those reported in other countries and suggests poor adherence to the recommendation to adjust treatment at each medical visit according to possible changes in the level of risk. Thus, our analysis shows that few changes are made at follow-up visits in the low-risk patient, many of them being changes to the same regimen regardless of the level of clinical control. However, good clinical practice guidelines recommend considering ICS withdrawal in patients who do not have frequent exacerbations and have less than 300eosinophils/mm3. In contrast, a strong recommendation against ICS withdrawal in eosinophilic exacerbating patients is established on the basis of ICS withdrawal studies that have demonstrated a significant increase in the risk of exacerbations upon ICS withdrawal in patients with >300eosinophils/mm3,1–3 and that could justify the result of our analysis, eosinophilia as a factor related to ICS use in the low-risk patient.
Another consideration in the use of ICS is the asthma-COPD overlap phenotype.27 However, in our population of low-risk patients, only 7.8% had a diagnosis of asthma as an associated comorbidity. Therefore, patients without a concomitant diagnosis of asthma were also being treated with ICS, suggesting inappropriate frequent use of ICS in patients who achieve a low-risk level and good clinical control and who are not reevaluated at follow-up. However, it is important to remember that symptoms suggesting asthma and peripheral eosinophilia>300mm3 were independently associated with a greater probability of ICS prescription. These findings could justify, in part, maintenance treatment with ICS associated with bronchodilator, given the difficulty in establishing a differential diagnosis between asthma and COPD. Previous studies have demonstrated the difficulty of differential diagnosis in COPD,28 which could favor the frequent use of ICS, especially in patients with low-risk COPD. Another factor to take into account that could contribute to this discrepancy between actual practice and evidence-based therapeutic recommendations is that patients continue with their established treatment despite new therapeutic recommendations. The studies that have assessed the real-world prescription pathways that lead to triple therapy have indicated that the most common prescription pathway toward triple therapy was combined therapy (LABA plus ICS) and that a history of exacerbations had an influence on the pathway from LABA plus ICS toward triple therapy, regardless of GOLD classification.25,29 In our analysis, low-risk patients were characterized by an average follow-up time of 4.7 years and 26.9% had suffered a moderate exacerbation in the previous year. If we assume that the administration of triple therapy is optimal to achieve control in patients with COPD, that can result, once the patients have begun therapy with ICS plus LABA, in the following therapeutic option inevitably being triple therapy. However, to date, there is no proof of the superiority of triple therapy compared to long-acting bronchodilators in patients with a low risk of exacerbations.24 On the contrary, there is real-world evidence that supports the possibility of withdrawing regimens that contain ICS in patients that don’t require them.30,31
In our analysis, dual bronchodilator therapy was the most commonly prescribed inhaled medication in low-risk patients. A considerable increase is observed with respect to the results of EPOCONSUL 2015 (42.1% in 2021 compared to 26.3% in 2015),5 which is accompanied by a decrease in bronchodilator monotherapy (19.9% in 2021 compared to 34.8% in 2015).5 Clinical best practice guides recommend dual bronchodilator therapy for patients who remain symptomatic with limitations in their daily lives despite monotherapy, since it offers a functional benefit compared to monotherapy.1–3 In our study, a substantial percentage of patients with low-risk COPD received dual bronchodilator treatment despite having a mMRC dyspnea score of ≤1 with non-severe airflow obstruction (predicted FEV1≥50%) and the non-frequent exacerbator phenotype. However, several factors could contribute to the increase in the prescription of dual bronchodilator treatment, including the impact on quality of life reported by patients, which influences the medical assessment of disease severity. Some studies have shown that patients with moderate COPD can remain symptomatic despite monotherapy with LAMA.32,33 One consideration to take into account is that this is an audit of patients being followed up in consultation, so that maintenance prescription patterns are evaluated, and therefore it is possible that the combination of bronchodilators was recommend due to greater dyspnea, and thanks to the treatment they achieve better control and criteria of low risk.
In our population, a substantial percentage of low-risk patients had a CAT score above 10 (40.7%), an assessment that takes into account not only dyspnea, but also other symptoms of COPD and health status. It is important to point out that, in some cases, the discrepancy between current therapeutic recommendations and real clinical practice could be due to patients’ individual needs and comorbidities.34
Triple therapy continues to be the most commonly prescribed medication in patients with high-risk COPD in outpatient respiratory clinics in Spain. This is a therapeutic option that has been recommended for years as part of a strategy to intensify treatment in COPD. It is currently recommended for symptomatic patients with a high risk of exacerbations1–3 based on several clinical trials that have shown that it brings added benefits compared to monotherapy and dual therapies, improving efficacy with regard to a decrease in exacerbations and hospitalization rates.35
In the present study, high-risk patients treated with triple therapy versus dual bronchodilator therapy were more symptomatic and had greater airflow obstruction, with worse clinical control of their COPD, in addition to being more frequent exacerbators. In our analysis, symptoms suggestive of asthma, dyspnea ≥2 and severe airflow obstruction were independently associated with a greater probability of a prescription of triple therapy compared to dual bronchodilator therapy. These findings can explain, in part, the positioning of triple therapy in clinical practice in respiratory clinics as an escalation strategy in patients with a high clinical impact based on studies that have shown that triple therapy offers greater efficacy in improving lung function and respiratory symptoms.8–10 Thus, in our analysis, changing inhaled therapy at the visit was more frequent in high-risk patients, and the intensification or scaling was performed more frequently in patients with a high risk level versus those with a low risk level. High-risk patients treated with triple therapy versus dual bronchodilator therapy more frequently had a history of hospitalization for COPD exacerbations (29.9% versus 24.6%, p=0.001) and were more commonly frequent exacerbators (27% versus 19.2%, p<0.001), although triple therapy prescriptions did not differ between eosinophilic and non-eosinophilic exacerbators. Triple therapy was the most frequently prescribed option in high-risk patients for all clinical phenotypes, although it was less frequent in non-exacerbators (44.7%), non-eosinophilic exacerbators (62.6%) and eosinophilic exacerbators (61.4%). However, a history of exacerbations, eosinophil count in peripheral blood and smoking were not maintained as factors predicting triple therapy versus dual bronchodilator therapy. Other factors associated with the prescription of triple therapy were follow-up time and being treated at a university center.
In recent years, evidence has been gathered on the predictive role of peripheral eosinophilia in the clinical response to ICS in COPD,36,37 based on which scaling up to triple therapy is recommended in frequent exacerbators with >300eosinophils/mm3.1–3 In our population, between 21% and 23% of patients with COPD had >300eosinophils/mm3, a value similar to the range observed in previous studies (between 15% and 25%).12,38 In our analysis, we did not find differences in the average blood eosinophil count nor in the distribution of the eosinophil count in peripheral blood between high-risk patients treated with triple therapy versus dual bronchodilator therapy. This data suggests that long-term treatment intensification strategies in COPD follow-up with triple therapy are not related to blood eosinophil counts. In addition, our analysis shows that almost one third of patients with blood counts ≤100cells/μL were receiving triple therapy, despite this group being the least likely to benefit from these therapies.13 These data reflect the fact that the therapeutic attitude of pulmonologists in their clinical practice does not yet seem to be in line with what current guidelines recommend.
The main strengths of our study are its national scope and a comprehensive and systematic evaluation of the clinically relevant parameters. However, several methodological considerations must be taken into account in order to correctly interpret our results. The first is that this is a cross-sectional study and the data presented here is the historical evaluation of a specific clinical visit, so differences in the risk of exacerbations or complications compared to different therapeutic strategies cannot be evaluated. The second is that the clinical presentation of COPD in follow up varies, and it is therefore important to remember that the guidelines are general recommendations. Thirdly, participating centers were not selected randomly and hospitals’ participation was voluntary, depending on their previous experience with clinical studies on COPD and their interest in participating. Fourthly, any clinical audit has the intrinsic limitation of lost values (not available) regardless of the inclusion methodology and periodic supervision of the database. In our analysis, a considerable number of patients could not be included because they had missing values to define their risk level, although when their characteristics were compared with those of the included patients they did not differ greatly, which we believe limits their possible influence on the results. Despite these limitations, we believe that this dataset represents the largest sample from respiratory clinics in Spain, offering real-world data in patients with COPD.
ConclusionThis analysis shows an evaluation of pharmacological treatments inhaled therapy in the follow-up of COPD to help us understand how physicians use available drugs for patient care. The results show that few changes are made in the follow-up visits and treatment of COPD does not always follow the current guidelines, in particular with a frequent use of ICS in patients who meet criteria low-risk COPD, and use of triple therapy in high-risk COPD patients as an strategy in patients with high clinical impact.
Authors’ contributionsMCR, MM, JJLC, BAN, JJSC and JLRH form the study's Scientific Committee and designed the study and wrote the manuscript. MEFF did the statistical analysis. All authors contributed to data analysis, results interpretation, drafting and revising the paper, and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Institutional review board statementThe study was conducted in accordance with the Declaration of Helsinki and approved by the Clinical Ethics Committee at the Hospital Clínico San Carlos (Madrid, Spain; internal code 20/722-E).
Data availability statementThe data presented in this study are available on request from the corresponding author.
FundingThis study has been promoted and sponsored by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR). We thank Chiesi for its financial support in carrying out the study. The financing entities did not participate in the design of the study, data collection, analysis, publication, or preparation of this manuscript.
Conflicts of interestMCR has received speaker fees from AstraZeneca, Bial, Chiesi, CSL Behring, GlaxoSmithKline, Menarini, and Grifols, and consulting fees from GlaxoSmithKline and Bial. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, CSL Behring, Inhibrx, Ferrer, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon and Grifols and research grants from Grifols. JLLC has received honoraria for lecturing, scientific advice, participation in clinical studies or writing for publications for: AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, CSL Behring, Ferrer, Gebro, GlaxoSmithKline, Grifols, Menarini, Megalabs, Novartis and Rovi. BAN reports grants and personal fees from GSK, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Chiesi, non-financial support from Laboratorios Menarini, grants, personal fees and non-financial support from AstraZeneca, personal fees from Gilead, personal fees and non-financial support from MSD, personal fees from Laboratorios BIAL, personal fees from Zambon, outside the submitted work; in addition, Dr. Alcázar-Navarrete has a patent P201730724 issued. JJSC has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, FAES, GlaxoSmithKline, Menarini and Novartis, and consulting fees from Bial, Chiesi and GSK, and grants from GSK. JLRH has received speaker fees from Bial, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Zambon and Grifols, and consulting fees from Bial.