After more than two years of pandemic, COVID-19 infection continues to cause great morbidity and mortality worldwide.1 The clinical course is heterogeneous, from asymptomatic cases to severe pneumonia with acute respiratory distress.2 Several factors associated with a worse prognosis have been identified, including advanced age, male sex, ethnicity, and comorbidities such as arterial hypertension, diabetes mellitus, cardiovascular disease, obesity, neoplastic disease.3–5 Similarly, smoking habit and underlying chronic respiratory diseases, especially chronic obstructive pulmonary disease, can also favour a more severe course of COVID-19.6,7
Interstitial lung diseases (ILDs) comprise a heterogeneous group of diseases in which the alveolar walls are infiltrated by various combinations of inflammatory cells and fibrosis. Idiopathic pulmonary fibrosis (IPF) is the most common chronic fibrotic ILD, progressive from time of diagnosis, with gradual decline in lung function and poor prognosis. Other subtypes of ILDs, which generally have a greater inflammatory component, often have a more benign course than IPF.8 However, a proportion of these ILDs may present progressive pulmonary fibrosing during their evolution that leads to decline in lung function, worsening of respiratory symptoms, deterioration of quality of life, and increased mortality, as occurs in IPF.9
The impact of COVID-19 infection on patients with pre-existing ILD is of increase interest, since in these patients with already impaired lung function, respiratory infections can trigger an acute exacerbation of the disease or a subsequent clinical and functional worsening, particularly in the case of IPF.10 Clinical experience suggests that progression of pre-existing ILD also occurs in those who survive the infection, although further studies are needed in this respect to make this assertion.11
We conducted an observational multicentre case series study of patients over 18 years old with a previous diagnosis of ILD (IPF or other ILDs), who presented COVID-19 infection between March 1st 2020 and September 30th 2020. The objective of the study was to assess the effects of COVID-19 in patients with pre-existing ILDs.
A survey was conducted in multidisciplinary ILD units from the Spanish territory. A specific collection sheet was sent. The centres were contacted and invited to participate through the ILD group of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR).
Epidemiological and clinical variables were collected, including: age, sex, ethnicity, body mass index, ILD subtype and their specific treatment, forced vital capacity (FVC) and diffusing capacity of carbon monoxide (DLCO), severity of COVID-19 infection and its management. The study was approved by the Drug Research Ethics Committee of Hospital Universitario de la Princesa (Madrid, 06-16-20, act CEIm 14/20).
A descriptive and comparative analysis of sociodemographic and clinical variables was performed, looking for predictors of mortality and comparing mortality between three distinct groups of ILDs: (1) IPF; (2) ILD associated with connective tissue diseases (CTD-ILD); and (3) other ILDs.
For continuous variables, results were expressed as mean and standard deviation (SD). The Shapiro–W Kolmogorov–Smirnov test was performed to check the normality of the continuous variables. Homoscedasticity was verified using Levene's test. When the distributions were normal and homoscedastic, a parametric test (t-test) was performed. In the event that at least one of these two assumptions did not occur, a nonparametric test (Mann–Whitney U test) was performed. In the case of categorical variables, the comparison of proportions was verified using the Chi-square test or Fisher's exact test, whenever necessary. A crude multivariate logistic regression analysis was performed in order to obtain the odds ratios and their 95% CIs, as estimators of mortality in patients with ILD. In all the analyses performed, a p-value below 0.05 was considered statistically significant. The entire statistical analysis was performed using the software “R”.
A total of 137 patients were included, from 20 centres from several Spanish regions. The mean age was 68.5 years old (±11.4 SD), with majority of male (88 men, 64.2%), predominantly Caucasian (89.1%) and in lower proportion, Black and Latino (1.5% and 6.6%, respectively). Approximately 61% of patients were current or former smokers, and the mean BMI was 27.89kg/m2 (±4.35 SD). The most frequent comorbidities were arterial hypertension (n=77; 56.6%), dyslipidaemia (n=72; 52.9%) and diabetes mellitus (n=38; 27.7%).
Regarding the baseline treatment of all ILDs patients, 19 patients (41.3%) had long-term oxygen therapy (LTOT), 37 (80.4%), antifibrotic therapy, 8 (17.4%), oral corticosteroids, and other 8 patients (17.4%), a combination of immunosuppressive therapy and oral corticosteroids, whereas 9 patients (19.6%) did not have any specific treatment. Approximately half of the patients on corticosteroids (46.2%) were receiving low doses (<10mg/day of equivalent prednisone), more than 6 months prior to diagnosis of COVID-19. The diagnosis of COVID-19 infection was made essentially by RT-PCR test (106 patients, 83.5%), whereas in 5.6% it was made by serology, and in 15 patients (10.9%), clinical diagnosis was made without microbiological confirmation.
The distribution among the three established subgroups of ILDs was: (1) IPF (n=46; 33.6%); (2) CTD-ILD (n=30; 21.9%); and (3) other ILDs (n=61; 44.5%). The mean FVC% of predictive for each group was 73.7% (±16.8 SD), 80.9% (±18.7 SD), and 77.9% (±20.0 SD), respectively (Table 1).
Comparison of the Evolution of COVID-19 in the Three Differentiated ILD Groups: IPF, CTD-ILD, and Other ILDs.
| IPF (n=46) | CTD-ILDB(n=30) | Other ILDC(n=61) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 71.0 (±8.6) | 65.3 (±11.1) | 68.2 (±13.0) | 0.128 |
| Gender (men) | 36 (78.3%) | 12 (40.0%) | 40 (65.6%) | 0.009* |
| Ethnicity (Caucasian) | 45 (97.8%) | 22 (73.3%) | 56 (91.8%) | 0.006* |
| BMI | 26.9 (±4.4) | 27.7 (±4.8) | 28.3 (±5.5) | 0.093 |
| Smoking habit (non-smoker) | 33 (71.7%) | 14 (46.7%) | 36 (59.0%) | 0.180 |
| More frequent comorbidities | ||||
| Arterial hypertension | 24 (52.2%) | 18 (60.0%) | 35 (57.4%) | 0.916 |
| Dyslipidaemia | 27 (58.7%) | 14 (46.7%) | 31 (50.8%) | 0.756 |
| Diabetes mellitus | 13 (28.3%) | 8 (26.7%) | 17 (27.9%) | 0.999 |
| Pulmonary function testsA | ||||
| FVC (litres) | 2.6 (±0.9) | 2.6 (±0.7) | 2.7 (±1.1) | 0.871 |
| FVC% pred | 73.7 (±16.8) | 80.9 (±18.7) | 77.9 (±20.0) | 0.259 |
| FVC ≤80% pred | 30 (66.7%) | 17 (63.0%) | 33 (55.0%) | 0.672 |
| DLCO pred | 48.4 (±20.8) | 53.9 (±11.6) | 59.7 (±19.6) | 0.014* |
| DLCO ≤40% pred | 16 (38.1%) | 2 (8.0%) | 9 (16.1%) | 0.016* |
| Baseline treatment for ILD | ||||
| Long term oxygen therapy | 19 (41.3%) | 2 (6.7%) | 10 (16.4%) | 0.002* |
| Antifibrotics | 37 (80.4%) | 1 (3.33%) | 4 (6.6%) | <0.001* |
| Corticosteroids | 8 (17.4%) | 21 (70.0%) | 32 (52.5%) | <0.001* |
| Immunosuppressants combined with corticosteroids | 8 (17.4%) | 26 (86.7%) | 33 (54.1%) | <0.001* |
| None | 9 (19.6%) | 4 (13.3%) | 26 (42.6%) | 0.011* |
| Management of COVID infection | ||||
| Outpatient | 6 (13.0%) | 2 (6.7%) | 10 (16.4%) | 0.434 |
| Conventional hospitalisation | 33 (71.7%) | 12 (40.0%) | 39 (63.9%) | 0.018* |
| Ventilatory support (ICU or IRCU)A | 7 (15.2%) | 16 (53.3%) | 12 (19.7%) | <0.001* |
| Outcome | ||||
| Exitus | 16 (34.8%) | 9 (30.0%) | 20 (32.8%) | 0.979 |
APulmonary function tests: determined by pulmonary function tests 6 months prior to COVID diagnosis. The study includes data from the majority of patients: FVC of 132 patients (absolute value and %), and DLCO of 123 patients.
BCTD-ILD (connective tissue disease-associated interstitial lung disease): 13 cases of rheumatoid arthritis (RA); 7 Sjögren's syndrome (SS); 2 systemic lupus erythematosus (SLE); 6 systemic sclerosis (SSC); 2 dermatomyositis (DM); polymyositis (PM).
COther ILDs: 8 chronic hypersensitivity pneumonitis (CHP); 4 combined pulmonary fibrosis and emphysema (CPFE); 3 smoking-related ILD; 8 unclassifiable ILD (U-ILD); 1 asbestosis; 1 diffuse idiopathic pulmonary neuroendocrine cell hyperplasia (DIPNECH); 1 Langerhans cell histiocytosis (LCH); 4 interstitial pneumonia with autoimmune features (IPAF); 1 chronic eosinophilic pneumonia (CEP); 1 bronchiolocentric interstitial pneumonia; 12 nonspecific interstitial pneumonia (NSIP); 5 cryptogenic organizing pneumonia (COP); 10 sarcoidosis; 1 hereditary hemorrhagic telangiectasia (HHT or Rendu–Osler–Weber syndrome).
CPulmonary function tests: determined by pulmonary function tests 6 months prior to COVID diagnosis. The study includes data from the majority of patients: FVC of 132 patients (absolute value and %), and DLCO of 123 patients.
Body mass index (BMI); CTD-ILD (connective tissue disease-associated interstitial lung diseases); interstitial lung disease (ILD); idiopathic pulmonary fibrosis (IPF); forced vital capacity (FVC); diffusing capacity of carbon monoxide (DLCO); intensive care unit (ICU); intermediate respiratory care unit (IRCU).
Our study showed that the vast majority of patients required hospital admission (119 patients, 86.9%): 84 (61.3%) in a conventional hospitalization ward, and 35 (25.5%) in the ICU or intermediate respiratory care unit (IRCU). In particular, patients with IPF received mechanical ventilation only in 15.2% of cases, a significantly lower percentage comparing with the other two ILD subgroups, (Table 1) which could be explained by the caution or not use of MV in IPF patients due to poor outcomes.12,13
Consistently, according to published studies, patients with pre-existing ILD and COVID-19 infection develop more severe forms, requiring more frequently hospital admission, oxygen therapy, ICU admission and mechanical ventilation (MV).14–16 Additionally, they have significantly higher mortality,14,15,17,18 with an adjusted risk of death up to four times higher.19 Particularly, the study by Gallay et al.20 describes a probability of mortality of 35% in patients with IPF, compared to 19% in other types of ILDs.
Finally, 45 (37.8%) hospitalized patients died, three of them after being discharged up to the time of the recruitment cutoff. Patients with IPF presented higher mortality compared to “CTD-ILDs” and “other ILDs” (34.8% versus 30% and 32.8%, respectively, p=0.979) (Table 1), although these differences did not reach statistical significance, probably due to the low sample size, which is the main limitation of our study. Moreover, in the crude and multivariable analysis, some factors showed a trend to association with a higher risk of mortality: previous diagnosis of IPF, older age (over 60 years), history of arterial hypertension or diabetes mellitus, impaired baseline lung function (FVC≤80%, DLCO≤40%) and the use of LTOT. However, statistically significant differences were only found for LTOT (p=0.027).
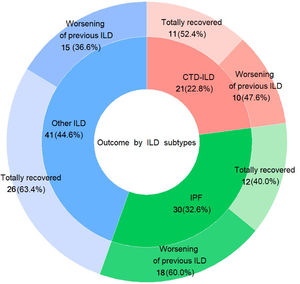
Regarding the outcome after acute COVID-19 infection, patients who required hospitalisation had a significantly higher frequency of clinical worsening of pre-existing ILD up to the time of the recruitment cutoff, compared to those managed on an outpatient basis (41 patients hospitalised -55.4%- vs. 2 patients -11.1%- outpatients, p=0.002), requiring either initiation of LTOT or increasing oxygen flow, or needing rehabilitation. Specifically, patients with IPF showed a trend towards worsening of pre-existing ILD (18 of 30 patients -60%-, p=0.12), while patients in the group “other ILDs” showed a trend towards complete recovery (26 of 41 patients -63.4-, p=0.124), although statistical significance was not reached. (Fig. 1) This is an interesting aspect of our study because so far, there is no data about post-infection outcomes in patients with pre-existing ILD. Therefore, further research is needed in this area.
In summary, due to increase morbimortality with COVID-19 infection, patients with ILD, particularly those with IPF, should receive adequate management and follow-up of their underlying disease and prioritized the preventive measures against COVID-19 infection.
Conflict of InterestsThe authors state that they have no conflict of interests.