We present 3 cases of Granulomatous Lymphocytic Interstitial Lung Disease (GLILD), in particular two of these patients started their clinical picture after a SARS-CoV-2 infection, additionally these had a more torpid evolution with respect to the case that was not associated with COVID-19 infection. Establishing the possibility of an association between SARS-CoV-2 infection and GLILD.
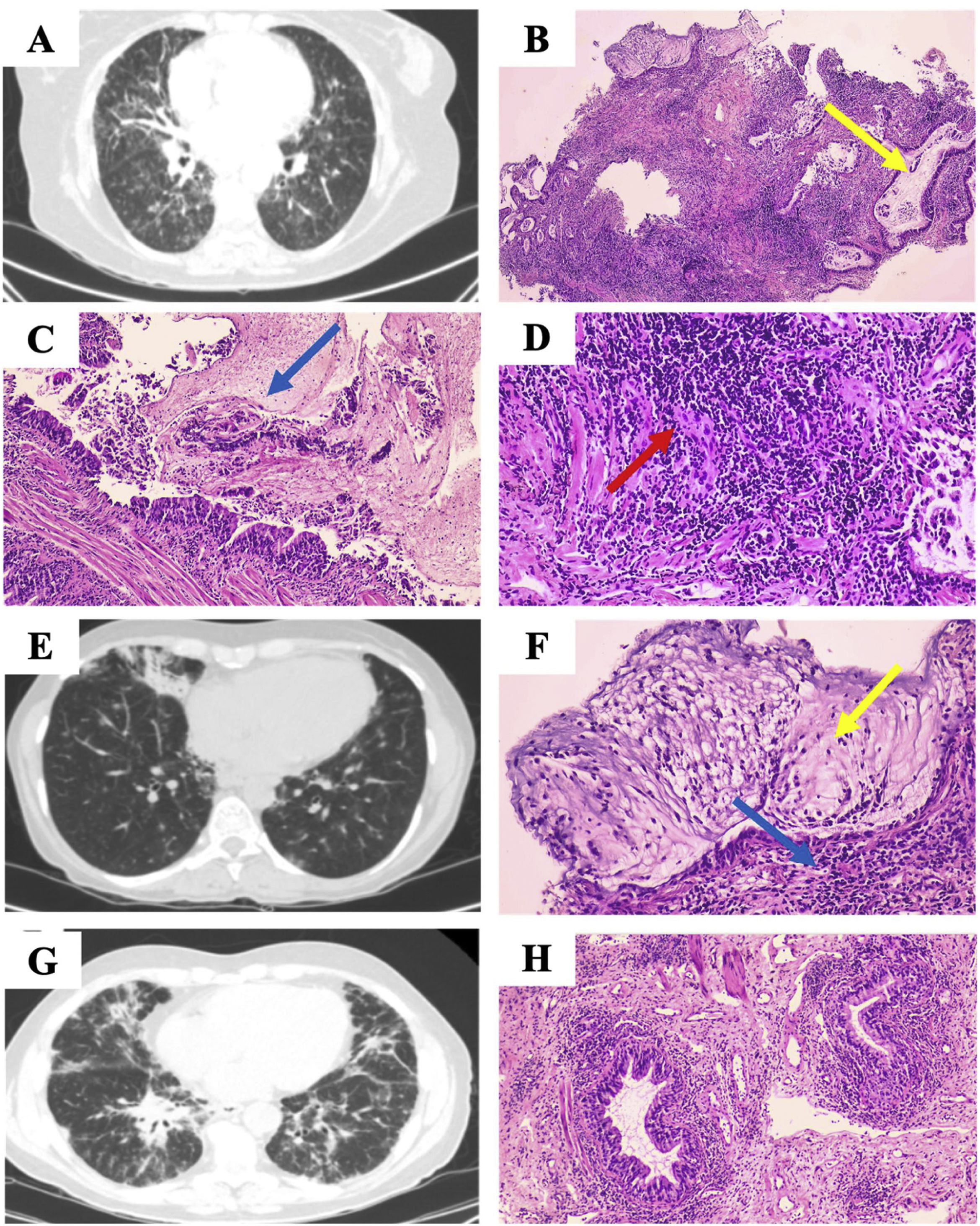
Case 158-Year-old woman diagnosed with stage I sarcoidosis 5 years ago at another center; referred for recurrent pneumonias. Chest computed tomography (CT) showed bronchiectasis and ground glass (Fig. 1A). The immunological study confirmed Common Variable Immunodeficiency (CVID), and intravenous immunoglobulin G (0.2g/kg/month) was started; transbronchial lung cryobiopsy (TBCB) showed bronchiolitis surrounded by lymphocytic infiltrate (Fig. 1B) and the diagnosis of Sarcoidosis was replaced by GLILD. The patient presented correct and stable evolution despite not receiving treatment with glucocorticoids.
(A) Extensive bilateral bronchiectasis and bronchioloectasis, tarnished glass, septal thickening and. (B) Lung parenchyma with abundant dilated bronchial structures with mucoid material (yellow arrow). (C and D) Intraluminal mucoid material shows intraluminal polymorphonuclear intraluminal polymorphs (blue arrow), and bronchiolar structures are surrounded by a notable lymphocytic inflammatory infiltrate (red arrow). (E) Hiliomediastinal adenopathies (up to 18mm, previously 15mm), bronchiectasis, nodular lesions and ground glass. (F) Bronchial structures surrounded by lymphocytic infiltrate (yellow arrow), mucoid material with presence of neutrophils (blue arrow). (G) Reticulonodular pattern with predominant distribution in bases, paratracheal (12mm) and subcarinal (10mm) adenopathy. (H) Peribronchial lymphocytic infiltrate.
To emphasize that, at the time the patient did not undergo a biopsy to confirm the diagnosis of Sarcoidosis.
Case 248-Year-old woman with CVID refers that after SARS-CoV-2 pneumonia has presented recurrent respiratory infections. Thoracic CT showed bronchiectasis and hiliomediastinal adenopathies; the patient refused invasive studies, opting to administer Immunoglobulin G (0.2g/kg/month) and inhaled Tobramycin. After three years of follow-up, the adenopathies increased in size and there was a newly appearing interstitial infiltrate (Fig. 1E), which led to the acceptance of linear ultrasound bronchoscopy plus fine needle puncture of subcarinal adenopathy, which was inconclusive. TBCB was performed (Fig. 1F), revealed lymphoid hyperplasia confirming the suspicion of GLILD, prescribing 2 cycles of prednisone (30mg/day in a descending pattern) for one year, later discontinued due to radiological stability for one year.
Case 366-Year-old male with recurrent respiratory infections and admitted one year ago for SARS-CoV-2 pneumonia; daughter carrying MSH5 genetic variant and CVID. Thoracic CT showed reticulonodular pattern (Fig. 1G), transbronchial lung biopsy (TBB) revealed lymphoid infiltrate (Fig. 1H), and immunological study detected IgG and IgA deficiency; two allelic variants of the MSH5 gene were identified. The diagnosis of CVID-GLILD was established administered gammaglobulin, prednisone (60mg/day at a descending rate) and Azathioprine (2mg/day), The latter two were suspended after 2 years after maintaining clinical–radiological stability. One year after discontinuation of immunosuppressants, the patient developed nodular lymphocyte-predominant Hodgkin lymphoma.
GLILD occurs in approximately 20% of patients with CVID,1 and the factors that favor the development of this pulmonary complication in this subgroup are unknown. Some cases of CVID-GLILD have a low T-lymphocyte count and an increased risk of developing other lymphoproliferative disorders,2 with infections by lymphotropic viruses as a possible cause.2 In turn, certain patients with CVID overproduce cytokines such as tumor necrosis factor alpha (TNF-α), and this would favor the formation of granulomas.3 It is noteworthy that two of our cases debuted with pulmonary involvement after COVID-19 infection. Although none of them presented lymphopenia, a probable hypothesis could be the exaggerated production of cytokines by CVID added to the elevation of TNF-α due to SARS-CoV-2 infection4; although studies are needed to prove this association.
Use of artificial intelligence to generate textsThe authors declare that they have not used any type of generative artificial intelligence in the writing of this manuscript or for the creation of figures, graphs, tables or their corresponding captions or legends.
FundingThis research has not received any specific grants from agencies in the public, commercial or for-profit sectors.
Conflict of interestsThe authors state that they have no conflict of interests.