The diagnosis of latent tuberculous infection (LTI) by IGRA continues to generate debate. Experience in the simultaneous use of 2 IGRA tests is scant. The aim of this study was to compare the results of 2 versions of QuantiFERON-TB Gold (In-Tube/Plus) with those of T-SPOT.TB, and to analyse the effectiveness of a dual strategy (T-SPOT.TB + QTF) for the diagnosis of LTI in an immunosuppressed population.
MethodsWe conducted a prospective study (May 2015–June 2017) that included 2999 immunosuppressed patients and/or candidates for biologics, in whom 2 simultaneous IGRA tests were performed: Group 1 (1535 patients): T-SPOT.TB + QuantiFERON-TB Gold-In-Tube (QTF-GIT); Group 2 (1464 patients): T-SPOT.TB + QuantiFERON-TB Gold Plus (QTF-Plus.
ResultsThe concordance between QTF-GIT and T-SPOT.TB was 83.19% (κ = 0.532). The percentage of positive, negative, and indeterminate results were, respectively: 14.33% vs. 17.06%; 82.41% vs. 74.46%; and 3.25% vs. 8.46%. The concordance between QTF-Plus and T-SPOT.TB was 87.56% (κ = 0.609). The percentage of positive, negative, and indeterminate results were, respectively: 15.02% vs. 15.36%; 82.92% vs. 79.37%; and 2.04% vs. 5.25%. Discrepancies between T-SPOT.TB and QTF-Plus were 12.43%, suggesting that 103 patients were positive and another 79 were negative due exclusively to 1 of the 2 IGRAs.
ConclusionsGreater concordance was found between QTF-Plus and T-SPOT.TB than between QTF-GIT and T-SPOT.TB. However, we believe that the proportion of discrepancies between T-SPOT.TB and QTF-Plus is sufficiently important from a clinical point of view to justify the simultaneous use of 2 IGRA in this specific patient group.
El diagnóstico de la infección tuberculosa latente (ITL) mediante IGRA sigue generando debate. La experiencia empleando dos pruebas IGRA de manera simultánea es escasa. El objetivo de este estudio es comparar los resultados de dos versiones de QuantiFERON-TB Gold (In-Tube/Plus) con los de T-SPOT.TB y analizar la eficacia de esta estrategia dual (T-SPOT.TB + QTF) para el diagnóstico de la ITL en población con alguna condición inmunosupresora.
MétodosEstudio prospectivo (mayo 2015–junio 2017) que incluye 2.999 pacientes inmunodeprimidos y/o candidatos a terapias biológicas, a los que se les realizó de manera simultánea dos IGRA: grupo-1 (1.535 pacientes): T-SPOT.TB + QuantiFERON-TB Gold-In-Tube (QTF-GIT); grupo-2 (1.464 pacientes): T-SPOT.TB + QuantiFERON-TB Gold Plus (QTF-Plus).
ResultadosLa concordancia entre QTF-GIT y T-SPOT.TB fue del 83,19% (κ = 0,532). Las proporciones de resultados positivos, negativos e indeterminados fueron, respectivamente: 14,33 vs. 17,06%; 82,41 vs. 74,46%; y 3,25 vs. 8,46%. La concordancia entre QTF-Plus y T-SPOT.TB fue del 87,56% (κ = 0,609). Las proporciones de resultados positivos, negativos e indeterminados fueron, respectivamente: 15,02 vs. 15,36%; 82,92 vs. 79,37%; y 2,04 vs. 5,25%. Las discordancias entre T-SPOT.TB y QTF-Plus fueron del 12,43%, que implicaban que había 103 pacientes positivos y otros 79 pacientes negativos a expensas exclusivamente de uno de los dos IGRA.
ConclusionesSe evidenció una mayor concordancia entre QTF-Plus y T-SPOT.TB que entre QTF-GIT y T-SPOT.TB. Sin embargo, creemos que la proporción de resultados discordantes entre T-SPOT.TB y QTF-Plus es lo suficientemente relevante clínicamente como para justificar el empleo simultáneo de dos IGRA en este grupo específico de pacientes.
Despite effective treatment, tuberculosis (TB) remains the leading cause of death from infection worldwide. As the infected population (one quarter of the world’s population) constitutes the largest reservoir for developing active TB, WHO recommends the use of latent TB infection (LTBI) screening within the framework of its “End-TB” strategy, which sets targets of 95% reduction in tuberculosis deaths and 90% reduction in the global incidence in 2035 compared to the 2015 baseline1. A raft of measures will be required to achieve these figures, including, importantly, the identification and management of individuals with LTBI.
LTBI is diagnosed using immunological tests that detect an individual’s sensitization to antigens expressed by Mycobacteria tuberculosis in the absence of clinical and/or radiological findings consistent with active tuberculous disease. There is no gold standard for the diagnosis of LTBI, so the tuberculin skin test (TST) and the interferon-gamma release assay (IGRA), alone or in combination, are the techniques commonly used to indirectly diagnose this infection. TST has some well-known limitations2, including false negatives in immunocompromised patients and false positives in subjects vaccinated with BCG or sensitized to non-tuberculous mycobacteria. IGRAs, which measure the production of interferon gamma (IFN-γ) in response to specific antigens CFP-10 and ESAT-6 were introduced just over 10 years ago. Two types of IGRA are available: QuantiFERON™ (QTF) (Qiagen) and T-SPOT.TB™ (Oxford Immunotec).
The interpretation of IGRA results is objective, although sources of variability in these techniques can influence the results3. In general, the use of IGRAs is recommended in low-incidence countries due to their high specificity. However, neither IGRA nor TST differentiate between LTBI and active tuberculosis, and their positive predictive value of disease progression is less than 5%4–6.
In Spain, 2 consensus documents have been published that specifically address immunocompromised patients: the guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEMMI) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR)7; and a multidisciplinary document on the prevention and treatment of tuberculosis in patients who are candidates for biological therapies8. Both recommend screening for LTBI with TST and IGRA. Guidelines from the American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention recommend dual testing (TST + IGRA) in cases with a high probability of infection and/or progression to disease2. These recommendations are based on evidence accumulated using the QuantiFERON TB Gold-In-Tube™ (QTF-GIT) version.
The new QuantiFERON TB Gold Plus (QFT-Plus, QuantiFERON fourth generation) differs from QTF-GIT in that it incorporates a second tube containing modified antigens to stimulate the response of CD8+ T lymphocytes. Previous studies have shown increased sensitivity in immunosuppressed patients9 and in elderly patients10. It has been postulated that a greater release of IFN-γ in the TB2 tube compared to the TB1 tube could be due to recent infection.
To date, studies with QTF-Plus have been carried out mainly in patients with active tuberculosis11,12, in tuberculosis contact tracing13, and in LTBI screening14–16; in these populations, the yield of QTF-Plus was equivalent to that of QTF-GIT. Less evidence has been published in immunocompromised patients17.
The objectives of this study were to compare the results of QTF-GIT and QTF-Plus, to compare these with T-SPOT.TB results, and to evaluate the benefit of an LTBI strategy that uses both IGRAs simultaneously in immunosuppressed patients and/or candidates for biological therapies.
MethodsThis was a prospective study that included patients who were tested simultaneously with both IGRAs (group 1: T-SPOT.TB and QTF-GIT from May 2015 to June 2016; group 2: T-SPOT.TB and QTF-Plus from July 2016 to June 2017) due to a immunosuppressive condition or a high individual risk of developing tuberculosis, including immunosuppressed patients, individuals with chronic immune-mediated inflammatory diseases who were candidates for biological therapies, patients with hematological diseases or HIV-positive individuals.
This observational study received a favorable opinion from the Research Ethics Committee of the Principality of Asturias, and that ethics committee also approved the informed consent waiver.
In Asturias (1 million inhabitants; TB incidence rate of 10 cases/100,000 inhabitants), the diagnosis of tuberculosis infection by IGRA is performed centrally in the Regional Mycobacteria Reference Unit of the Hospital Universitario Central de Asturias, in coordination with the microbiology laboratories of the Health Service of the Principality of Asturias. On May 21, 2015, the Directorate-General for Public Health (Ministry of Health of the Principality of Asturias) published Circular 04/2015, updating the procedures for the diagnosis of tuberculosis infection in Asturias18, which recommends that two IGRA techniques be performed simultaneously (QuantiFERON and T-SPOT.TB) in immunocompromised patients and/or candidates for biological therapies.
All patients included in our study were tested with two IGRAs: ELISA (enzyme-linked immunosorbent assay)-based QuantiFERON TB Gold, which measures the amount of IFN-γ released into plasma after stimulation with specific antigens. We used the version available in each of the analyzed periods: (1) from May 2015 to June 2016: QTF-GIT, which consists of 3 tubes: negative control (nil), positive control (mitogen) and ESAT-6, CFP-10 and TB7.7 antigen tube (antigen); and (2) from July 2016 to June 2017: QFT-Plus, which consists of 4 tubes: nil, mitogen, TB1 tube (contains ESAT-6 and CFP-10 peptides optimized to generate response from CD4+ helper T lymphocytes) and TB2 tube (same peptides optimized to induce responses of both CD4+ and CD8+ cytotoxic T lymphocytes); and T-SPOT.TB, based on an ELISPOT (enzyme-linked immunospot assay), which quantifies the number of effector T cells. To this end, mononuclear cells are extracted from peripheral blood and stimulated with the ESAT-6 and CFP-10 antigens individually in two separate wells (2 wells are also required for positive and negative controls); in the second period of the study, cells that have released IFN-γ, which will appear as spots at the bottom of the wells are quantified.
The recommendations of the manufacturers were followed when carrying out the IGRA tests. QTF results were interpreted as: positive, when the antigen response (antigen tube in QTF-GIT, TB1 or TB2 in QTF-Plus) minus the nil value was ≥0.35 IU/mL and ≥25% of the nil value; negative, when the antigenic response minus the nil value was <0.35 IU/mL or <25% of the nil value; inconclusive, when a) the nil value was >8 IU/mL or b) the antigenic response minus the nil value was <0.35 IU/mL or <25% of the nil value and the mitogen value was <0.5 IU/mL. T-SPOT.TB results were interpreted as: positive when the difference in spots and the nil control in any of the antigen-containing wells [ESAT-6 (panel A) or CFP-10 (panel B)] was ≥8 spots; negative when the difference was less than 8 spots; inconclusive, when (a) the nil control had >10 spots or (b) the mitogen had <20 spots.
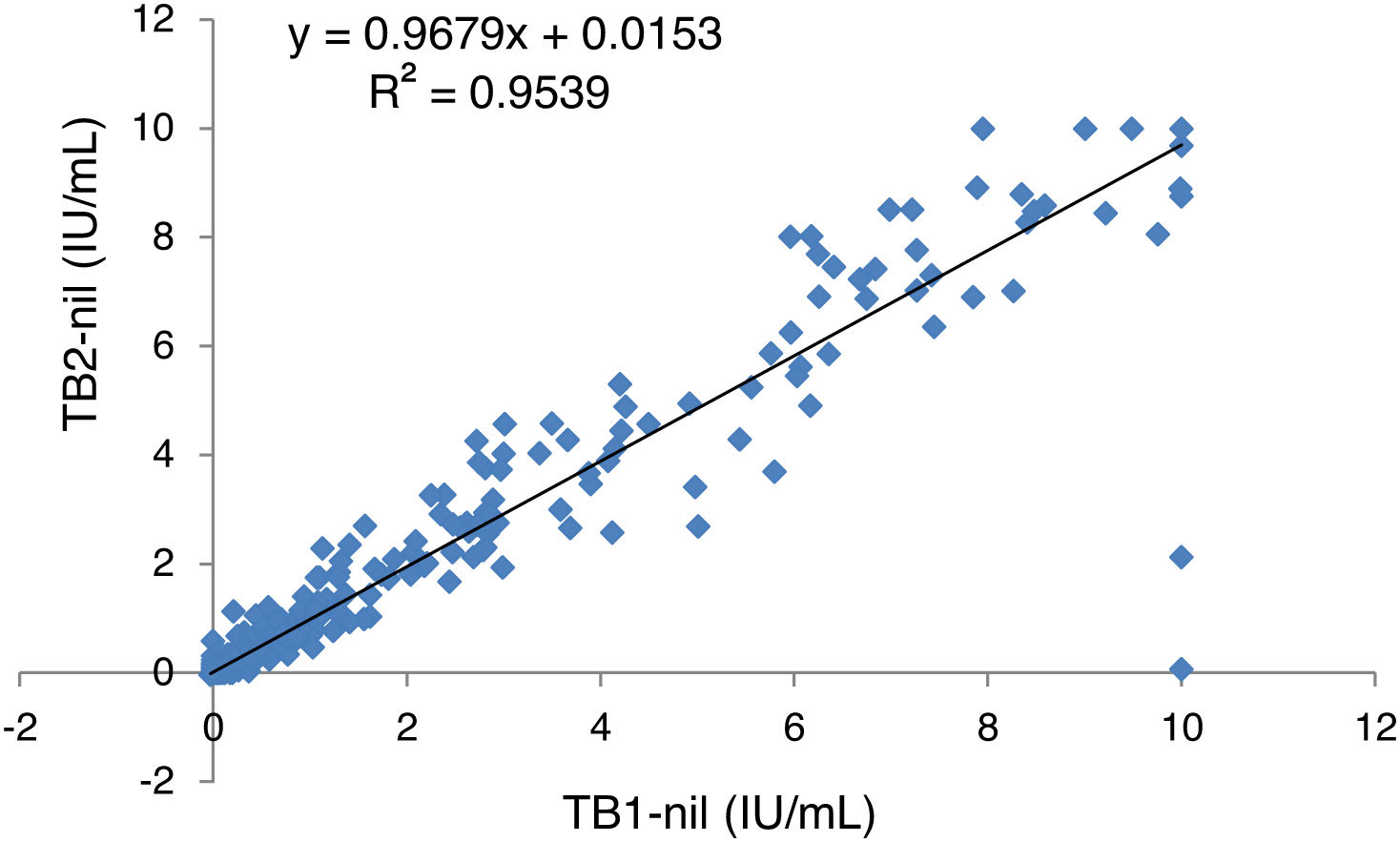
Statistical analyses were performed using R Studio. Cohen’s Kappa coefficient (κ) with 95% confidence intervals (CI) was used to evaluate the agreement between the two versions of QTF and T-SPOT.TB. The κ were interpreted according to the classification of Landis and Koch: 0.01–0.20 indicates poor agreement; 0.21–0.40 fair; 0.41–0.60 moderate; 0.61–0.80 substantial; 0.80–1.00 almost perfect. The χ2 test was used to compare the demographic characteristics of the patients included in each study period. Linear regression analysis was used to represent the amount of IFN-γ released in both QTF-Plus tubes TB1 and TB2.
ResultsA total of 2999 patients were included, 1535 during the first period and 1464 during the second period of the study. The demographic characteristics of the subjects and the origin of the disease that caused the immunosuppression are shown in Table 1. No significant differences were found between the 2 groups of patients. Overall, patients with gastrointestinal (44%) and hematological (25–26%) diseases generated 70% of the diagnostic demand for dual IGRA studies (T-SPOT.TB + QTF-GIT or T-SPOT.TB + QTF-Plus).
Demographic characteristics of patients and origin of immunosuppression and/or indication of biological therapy.
| First period (n = 1535) | Second period (n = 1464) | p | |
|---|---|---|---|
| Sex | |||
| Men | 783 (51.0%) | 736 (50.3%) | NS |
| Women | 752 (49.0%) | 728 (49.7%) | NS |
| Age | |||
| 0−14 | 57 (3.7%) | 63 (4.3%) | NS |
| 15−24 | 83 (5.4%) | 96 (6.6%) | NS |
| 25−34 | 181 (11.8%) | 173 (11.8%) | NS |
| 35−44 | 329 (21.4%) | 253 (17.3%) | <0.005 |
| 45−54 | 311 (20.3%) | 347 (23.7%) | <0.05 |
| 55−64 | 326 (21.2%) | 274 (18.7%) | NS |
| >65 | 248 (16.2%) | 258 (17.6%) | NS |
| Origin of the immunosuppressive condition | |||
| Gastrointestinal | 676 (44.0%) | 653 (44.6%) | NS |
| Hematology | 389 (25.3%) | 383 (26.2%) | NS |
| Rheumatology | 177 (11.5%) | 154 (10.5%) | NS |
| Dermatology | 98 (6.4%) | 87 (5.9%) | NS |
| HIV | 55 (3.6%) | 61 (4.2%) | NS |
| Others | 140 (9.1%) | 126 (8.6%) | NS |
NS: not significant.
The percentage of patients with a positive IGRA result (QTF and/or T-SPOT.TB) during the first and second period was 20.06% (308/1535) and 18.71% (274/1464), respectively. The overall results of the IGRAs for each period are shown in Table 2.
Overall IGRA results for each period (QFT-GIT vs. T-SPOT.TB and QTF-Plus vs. T-SPOT.TB, respectively).
| First period | ||||
|---|---|---|---|---|
| QTF-GIT | Total T-SPOT.TB | |||
| Positive | Negative | Inconclusive | ||
| T-SPOT.TB | ||||
| Positive | 174 | 79 | 9 | 262 (17.06%) |
| Negative | 27 | 1089 | 27 | 1143 (74.46%) |
| Inconclusive | 19 | 97 | 14 | 130 (8.46%) |
| Total QTF-GIT | 220 (14.33%) | 1265 (82.41%) | 50 (3.25%) | 1535 |
| Second period | ||||
|---|---|---|---|---|
| QTF-Plus | Total T-SPOT.TB | |||
| Positive | Negative | Inconclusive | ||
| T-SPOT.TB | ||||
| Positive | 171 | 49 | 5 | 225 (15.36%) |
| Negative | 34 | 1107 | 21 | 1162 (79.37%) |
| Inconclusive | 15 | 58 | 4 | 77 (5.25%) |
| Total QTF-Plus | 220 (15.02%) | 1214 (82.92%) | 30 (2.04%) | 1464 |
QTF-GIT: QuantiFERON TB Gold In-Tube; QTF-Plus: QuantiFERON TB Gold Plus.
Overall agreement between QTF-Plus and T-SPOT.TB was higher than between QTF-GIT and T-SPOT.TB (87.57% [1282/1464], κ = 0.609 [95% CI 0.560–0.657] vs. 83.19% [1277/1535], κ = 0.532 [95% CI 0.486–0.578]), and also in the positive results (κ = 0.727 [95% CI 0.677–0.777] vs. κ = 0.671 [95% CI 0.619–0.722]).
With regard to QTF (GIT or Plus) and T-SPOT.TB discrepancies, the percentage of results between QTF (GIT or Plus) positive/T-SPOT.TB negative or inconclusive, and QTF (GIT or Plus) inconclusive/T-SPOT.TB negative, was practically identical in the first and second period (3% vs. 3.3% and 1.7% vs. 1.4%, respectively), whereas in the case of negative or inconclusive T-SPOT.TB/QTF (GIT or Plus) results, the percentage fell from 5.7% (QTF-GIT) to 3.6% (QTF-Plus) (Table 3).
Comparison of matched and mismatched results between IGRA during the two study periods (QTF-GIT/T-SPOT.TB vs. QTF-PLUS/T-SPOT.TB).
| QTF-GIT/T-SPOT.TB | QTF-Plus/T-SPOT.TB | |||
|---|---|---|---|---|
| N | % | N | % | |
| Overall agreement | 1277 | 83.19 | 1282 | 87.57 |
| QTF positive/T-SPOT.TB positive | 174 | 11.34 | 171 | 11.68 |
| QTF negative/T-SPOT.TB negative | 1089 | 70.94 | 1107 | 75.61 |
| QTF inconclusive /T-SPOT.TB inconclusive | 14 | 0.91 | 4 | 0.27 |
| Overall disagreement | 258 | 16.81 | 182 | 12.43 |
| QTF inconclusive /T-SPOT.TB negative or inconclusive | 46 | 3.00 | 49 | 3.35 |
| T-SPOT.TB positive/QTF negative or inconclusive | 88 | 5.73 | 54 | 3.69 |
| QTF negative/T-SPOT.TB inconclusive | 97 | 6.32 | 58 | 3.96 |
| T-SPOT.TB negative/QTF inconclusive | 27 | 1.76 | 21 | 1.43 |
| TOTAL | 1535 | 100 | 1464 | 100 |
QTF-GIT: QuantiFERON TB Gold In-Tube; QTF-Plus: QuantiFERON TB Gold Plus; QTF refers interchangeably to either of the two versions of QuantiFERON TB Gold (GIT or Plus).
The individualized results of QTF-Plus tubes TB1 (CD4+ stimulation) and TB2 (CD4+ and CD8+ stimulation) showed an agreement of 98.22% (1,438/1,464, κ = 0.927 [95% CI 0.899–0.955]). The number of positive results was slightly higher in TB1 than in TB2 [14.41% (211/1464) vs. 13.87% (203/1464)). The overall mismatch rate was 1.77% (26/1464), with 17 cases being TB1 positive/TB2 negative and being 9 TB1 negative/TB2 positive.
Linear regression analysis of IFN-γ values recorded in tubes TB1 and TB2 showed a high correlation between tube readings (Fig. 1). The mean IFN-γ (IU/mL) values recorded in samples with a positive result in at least one of the two tubes are presented individually in Table 4. In our series we found no case in which the differences between the readings of TB2 and TB1 were greater than 0.6 IU/mL (limit set to establish significant differences and exclude intra-test variability19).
Average IFN-γ values detected in QTF-Plus TB1 and TB2 tubes in QTF-positive samples in at least one of the two tubes. Broken down according to the result obtained with T-SPOT.TB.
| N | TB1 (IU/mL) | TB2 (IU/mL) | TB2-TB1a | |
|---|---|---|---|---|
| QTF-Plus positive/T-SPOT.TB positive | 171 | 4.26 [0.19−10.00] | 4.17 [0.03−10.00] | N/A |
| TB1 positive/TB2 positive | 155 | 4.60 [0.37−10.00] | 4.57 [0.36−10.00] | N/A |
| TB1 positive/TB2 negative | 11 | 1.29 [0.35−10.00] | 0.19 [0.03−0.34] | N/A |
| TB1 negative/TB2 positive | 5 | 0.28 [0.19−0.34] | 0.43 [0.35−0.68] | 0.15 |
| QTF-Plus positive/T-SPOT.TB negative or inconclusive | 49 | 1.78 [0.00−10.00] | 1.95 [0.25−10.00] | 0.17 |
| TB1 positive/TB2 positive | 39 | 2.14 [0.35−10.00] | 2.35 [0.35−10.00] | 0.21 |
| TB1 positive/TB2 negative | 6 | 0.41 [0.35−0.58] | 0.30 [0.25−0.32] | N/A |
| TB1 negative/TB2 positive | 4 | 0.25 [0.00−0.32] | 0.54 [0.35−0.75] | 0.29 |
| TOTAL positive TB1 and TB2 | TB1: 211 | 3.86 [0.35−10.00] | 3.96 [0.35−10.00] | 0.10 |
| TB2: 203 | ||||
N/A: not applicable.
Our study was conducted in a region that uses a diagnostic strategy of 2 simultaneous IGRAs (QTF + T-SPOT.TB) in immunosuppressed patients and/or candidates for biological therapies. We compared the results of two different versions of QTF and then compared them to those of T-SPOT.TB.
Overall, we observed a greater overall agreement between QTF-Plus and T-SPOT.TB, with a reduction in the number of discordant negative or inconclusive QTF/positive T-SPOT.TB results. In the specific case of positive IGRA results, the degree of agreement between QTF-Plus and T-SPOT.TB (κ = 0.727) was higher than between QTF-GIT and T-SPOT.TB during the first period (κ = 0.671).
In 2 recent studies comparing IGRA tests conducted in 154 patients with rheumatoid arthritis20 and in 92 renal transplant patients21, very similar degrees of agreement in positive IGRA results were found between QTF-Plus and T-SPOT.TB (κ = 0.51) and between QTF-GIT and T-SPOT.TB (κ = 0.48), although it should be noted that very few of the latter tests were performed, probably because the series included very limited patient numbers.
Despite this, we believe that our dual strategy of IGRA testing in immunocompromised patients is justified by the 12.43% mismatch rate between QTF-Plus and T-SPOT-TB, which would mean that 103 patients would test positive if only one IGRA was used (49 QTF-Plus; 54 T-SPOT.TB). Furthermore, another 79 patients had a negative result in one of the two IGRAs (58 QTF-Plus; 21 T-SPOT.TB), while the other test was inconclusive.
A higher proportion of inconclusive IGRA results has been reported in immunocompromised populations22,23. Latorre et al.24 found that negative results were more frequent in patients with Crohn’s disease compared to patients with rheumatic diseases and psoriasis. In our study, fewer inconclusive results were obtained with QTF-Plus than with QTF-GIT, an observation also reported in 2 other Japanese studies, in which inconclusive results fell from 5.2% with QTF-GIT to 0.7% with QTF-Plus20,21.
For this reason, we also believe that the simultaneous use of 2 IGRAs (QTF and T-SPOT.TB) improves the efficiency of LTBI screening in at-risk patients, as the inconclusive results of one IGRA largely correlated with a valid result in the other. In fact, inconclusive results were obtained simultaneously in both IGRAs in less than 1% of patients (14 patients in the first period and 4 in the second period).
Several authors have remarked on the high negative predictive value of IGRAs, and with respect to the positive predictive value, studies have found that T-SPOT.TB is a better predictor of progression to tuberculosis than QTF-GIT and TST5,6. One of the limitations of our study is the absence of patient follow-up data, so we cannot determine whether the lower number of mismatches between T-SPOT.TB and QTF-Plus suggests that QTF-Plus offers better predictive values of disease progression than those reported in the QTF-GIT literature. However, it should be borne in mind that, in the absence of a gold standard for tuberculosis infection, the reliability of a diagnostic test cannot be precisely established. In this regard, we believe that our study shows that in a significant number of immunocompromised patients, inconclusive (positive/negative) results are obtained between simultaneous IGRA tests, even in the absence of technical reasons (it should be pointed out that these tests include negative and positive controls).
As in other studies9,10, we found that the second TB2 tube in QTF-Plus also increased diagnostic yield. Regarding the specific CD8+ response associated with active tuberculosis23 and an increased exposure to M. tuberculosis in contact tracing studies13, a comparison of the mean IFN-γ values in our series showed that differences between TB2 and TB1 were never greater than 0.6 IU/mL (not attributable to intra-test variability19).
Similarly to other studies17,20, the degree of agreement between TB1 and TB2 in our series was excellent (κ = 0.927). Although, overall, we observed higher mean IFN-γ values in TB2, the difference was not significant and was not associated with any specific population group. In a recent study that included 317 immunocompromised patients17, researchers found a high degree of agreement between TB1 and TB2, but also observed a higher IFN-γ response in TB2, particularly in solid organ transplant candidates. This led them to conclude that QTF-Plus could improve the diagnosis of LTBI in these patients.
ConclusionsIn LTBI screening in an immunocompromised population, QTF-Plus results showed greater agreement with T-SPOT.TB compared to QTF-GIT. Moreover, there was a significant reduction in the rate of disagreement between QTF-Plus and T-SPOT.TB, and in inconclusive QTF results.
As the reason for the discrepancies between the different LTBI screening tests has not yet been established, our experience suggests that, whenever feasible, all available diagnostic tests should be performed in groups with a higher risk of disease, such as immunocompromised patients and/or candidates for biological therapies, in whom any positive results should be considered evidence of LTBI. Therefore, we believe that the rate of mismatched positive results between T-SPOT.TB and QTF-Plus, although lower than those observed with QTF-GIT, and the number of inconclusive results obtained with T-SPOT.TB (much higher than those obtained with QTF-Plus) is sufficiently large in the diagnosis of LTBI to support the simultaneous use of QTF-Plus and T-SPOT.TB in this specific group of patients.
Conflict of interestsThe authors state that they have no conflict of interests.
The SESPA Network of Microbiology Laboratories includes the Microbiology Services of: Hospital de Jarrio, Hospital Carmen y Severo Ochoa, Hospital Universitario San Agustín, Hospital Universitario de Cabueñes, Hospital de Jove, Hospital Grande Covián, Hospital Álvarez-Buylla, Hospital Valle del Nalón and Central University Hospital of Asturias (HUCA).