Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide [1,2]. Unfortunately, many COPD patients progress to advanced stages of the disease, where the symptomatic burden and functional limitations intensify considerably. This situation not only has a high emotional impact on patients and their caregivers but will also lead to a high consumption of health care resources and poorer survival rates [1,2]. Moreover, at this stage, medical care will face important challenges such as adequate symptomatic control or advance care planning. Identification of patients with COPD in advanced stages together with the implementation of comprehensive management strategies are fundamental aspects to optimize care, mitigate symptomatology and facilitate an adequate transition to palliative care models [2,3]. However, the available evidence in this field is very heterogeneous given the absence of a definition of advanced COPD [3,4]. Recently, in a Spanish consensus based on Delphi methodology, advanced COPD was defined as those patients with a severe airflow limitation with a forced expiratory volume in the first second (FEV1)<50% and at least two of the following criteria: dyspnea 3 or 4 of the modified Medical Research Council (mMRC) scale, chronic respiratory failure (CRF) or limitation of basic activities of daily living (ADL) [5]. In that consensus, exacerbations were excluded from the definition due to their irregular course over time and the high variability in their identification, stratification, and treatment, which made their inclusion impractical [5]. However, we now know that COPD exacerbations, especially those requiring hospitalization, represent a critical event in the evolution of the disease [6,7]. In addition to their high morbidity and mortality, frequent hospitalizations are indicative of a clinically fragile situation and increased patient vulnerability [6,7]. They often mark the beginning of a vicious circle in which each admission contributes to worsening baseline status and loss of autonomy [6–8]. Determining the proportion of patients who meet the criteria for advanced COPD, as well as its independent predictive capacity regarding events such as readmissions and mortality after severe exacerbation, could provide relevant information on its applicability in clinical practice. With these objectives in mind, we conducted the present study analyzing the frequency of patients in the SocioEPOC Cohort who met the criteria defined in the Spanish consensus document [5] and their association with readmissions at 3 months and mortality at 12, 24, and 36 months after hospital discharge.
The SocioEPOC cohort has been described in detail in previous publications [7,9]. Briefly, 545 COPD patients were recruited from two Galician university hospitals between 2018 and 2022. Eligible patients had a first admission during the study period with a primary diagnosis of a severe exacerbation, and were followed for three years. Sociodemographic, epidemiological, and clinical data (including the degree of dyspnea according to the mMRC scale), as well as functional status (the patient's ability to perform basic activities of daily living) and respiratory variables (such as FEV1 or use of long term domiciliary oxygen therapy) prior to admission were collected through medical record review and personal interviews with patients and their caregivers. Complete demographic, clinical, and social information was collected from the patients and is summarized in Table 1. To evaluate the predictive capacity of the advanced COPD consensus criteria, a multivariate analysis was performed using forward conditional logistic regression analysis in which, in addition to the combination of variables defining advanced COPD [5], other factors were included, such as age, sex, body mass index (BMI), CAT (COPD Assessment Test) questionnaire score as well as the number of comorbidities and hospitalizations for COPD exacerbation in the previous year. The multivariate analysis was carried out sixteen times (4 possible combinations×4 events analyzed). The percentages of each event and the adjusted risk ratios (RR) and their 95% confidence interval (95%CI) were recorded. The analysis was performed with the IBM SPSS Statistics 25 software package (IBM Corporation, Armonk, NY).
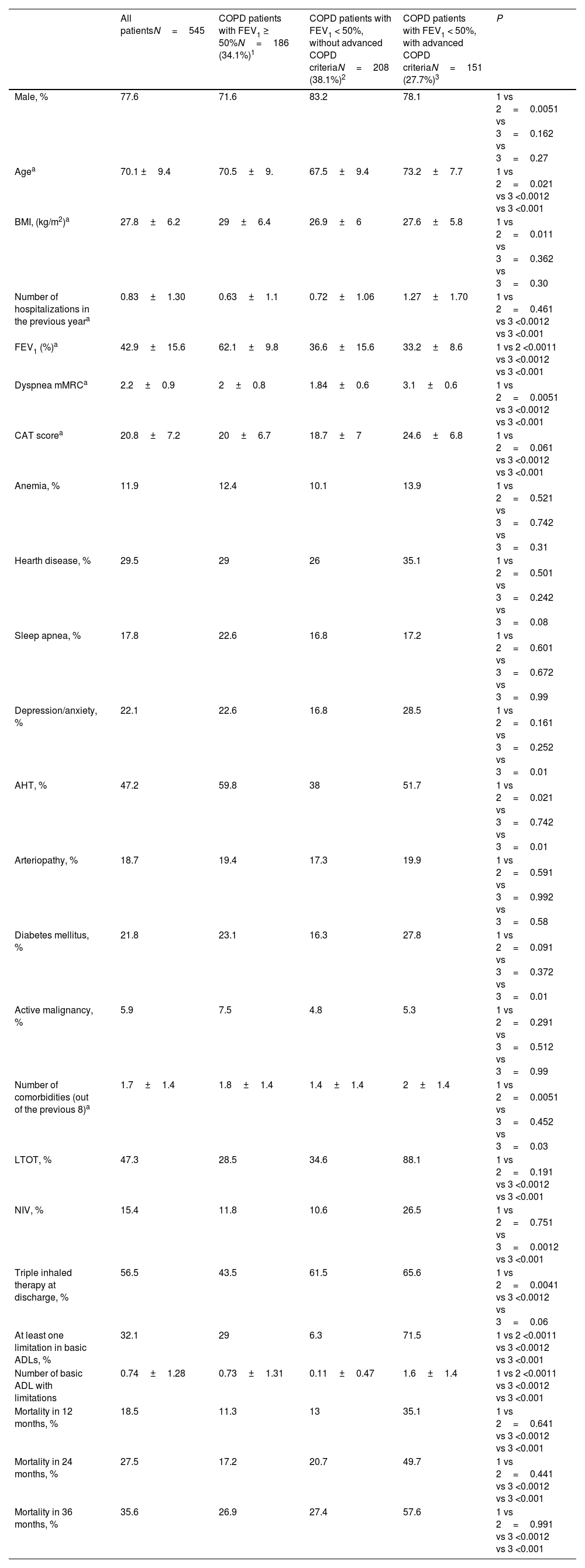
Comparative analysis of baseline characteristics and mortality in COPD subgroups from the SocioEPOC cohort.
| All patientsN=545 | COPD patients with FEV1 ≥ 50%N=186 (34.1%)1 | COPD patients with FEV1 < 50%, without advanced COPD criteriaN=208 (38.1%)2 | COPD patients with FEV1 < 50%, with advanced COPD criteriaN=151 (27.7%)3 | P | |
|---|---|---|---|---|---|
| Male, % | 77.6 | 71.6 | 83.2 | 78.1 | 1 vs 2=0.0051 vs 3=0.162 vs 3=0.27 |
| Agea | 70.1 ±9.4 | 70.5±9. | 67.5±9.4 | 73.2±7.7 | 1 vs 2=0.021 vs 3 <0.0012 vs 3 <0.001 |
| BMI, (kg/m2)a | 27.8±6.2 | 29±6.4 | 26.9±6 | 27.6±5.8 | 1 vs 2=0.011 vs 3=0.362 vs 3=0.30 |
| Number of hospitalizations in the previous yeara | 0.83±1.30 | 0.63±1.1 | 0.72±1.06 | 1.27±1.70 | 1 vs 2=0.461 vs 3 <0.0012 vs 3 <0.001 |
| FEV1 (%)a | 42.9±15.6 | 62.1±9.8 | 36.6±15.6 | 33.2±8.6 | 1 vs 2 <0.0011 vs 3 <0.0012 vs 3 <0.001 |
| Dyspnea mMRCa | 2.2±0.9 | 2±0.8 | 1.84±0.6 | 3.1±0.6 | 1 vs 2=0.0051 vs 3 <0.0012 vs 3 <0.001 |
| CAT scorea | 20.8±7.2 | 20±6.7 | 18.7±7 | 24.6±6.8 | 1 vs 2=0.061 vs 3 <0.0012 vs 3 <0.001 |
| Anemia, % | 11.9 | 12.4 | 10.1 | 13.9 | 1 vs 2=0.521 vs 3=0.742 vs 3=0.31 |
| Hearth disease, % | 29.5 | 29 | 26 | 35.1 | 1 vs 2=0.501 vs 3=0.242 vs 3=0.08 |
| Sleep apnea, % | 17.8 | 22.6 | 16.8 | 17.2 | 1 vs 2=0.601 vs 3=0.672 vs 3=0.99 |
| Depression/anxiety, % | 22.1 | 22.6 | 16.8 | 28.5 | 1 vs 2=0.161 vs 3=0.252 vs 3=0.01 |
| AHT, % | 47.2 | 59.8 | 38 | 51.7 | 1 vs 2=0.021 vs 3=0.742 vs 3=0.01 |
| Arteriopathy, % | 18.7 | 19.4 | 17.3 | 19.9 | 1 vs 2=0.591 vs 3=0.992 vs 3=0.58 |
| Diabetes mellitus, % | 21.8 | 23.1 | 16.3 | 27.8 | 1 vs 2=0.091 vs 3=0.372 vs 3=0.01 |
| Active malignancy, % | 5.9 | 7.5 | 4.8 | 5.3 | 1 vs 2=0.291 vs 3=0.512 vs 3=0.99 |
| Number of comorbidities (out of the previous 8)a | 1.7±1.4 | 1.8±1.4 | 1.4±1.4 | 2±1.4 | 1 vs 2=0.0051 vs 3=0.452 vs 3=0.03 |
| LTOT, % | 47.3 | 28.5 | 34.6 | 88.1 | 1 vs 2=0.191 vs 3 <0.0012 vs 3 <0.001 |
| NIV, % | 15.4 | 11.8 | 10.6 | 26.5 | 1 vs 2=0.751 vs 3=0.0012 vs 3 <0.001 |
| Triple inhaled therapy at discharge, % | 56.5 | 43.5 | 61.5 | 65.6 | 1 vs 2=0.0041 vs 3 <0.0012 vs 3=0.06 |
| At least one limitation in basic ADLs, % | 32.1 | 29 | 6.3 | 71.5 | 1 vs 2 <0.0011 vs 3 <0.0012 vs 3 <0.001 |
| Number of basic ADL with limitations | 0.74±1.28 | 0.73±1.31 | 0.11±0.47 | 1.6±1.4 | 1 vs 2 <0.0011 vs 3 <0.0012 vs 3 <0.001 |
| Mortality in 12 months, % | 18.5 | 11.3 | 13 | 35.1 | 1 vs 2=0.641 vs 3 <0.0012 vs 3 <0.001 |
| Mortality in 24 months, % | 27.5 | 17.2 | 20.7 | 49.7 | 1 vs 2=0.441 vs 3 <0.0012 vs 3 <0.001 |
| Mortality in 36 months, % | 35.6 | 26.9 | 27.4 | 57.6 | 1 vs 2=0.991 vs 3 <0.0012 vs 3 <0.001 |
Mean±standard deviation.
Abbreviations: ADL: activities of daily living (feeding, dressing, bathing, toilet use, going up/down stairs and chair transferring); AHT: arterial hypertension; BMI: body mass index; CAT: COPD Assessment Test; FEV1: Forced expiratory volume in the first second; LTOT: long term domiciliary oxygen therapy; mMRC: modified Medical Research Council; NIV: non-invasive ventilation.
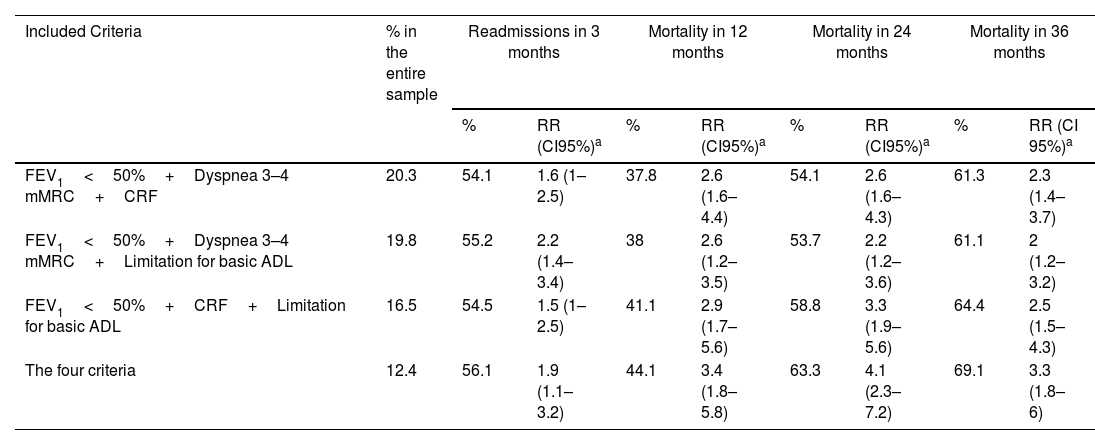
One hundred and fifty-one patients (27.7% of the cohort) met the criteria for advanced COPD. Of the total cohort, 36,9% were readmitted within 3 months; cumulative mortality was 18.5%, 27.5%, and 35.6% at 12, 24, and 36 months, respectively (baseline characteristics and mortality in COPD subgroups from the SocioEPOC Cohort are shown in Table 1). Table 2 describes the frequency of each of the events according to the possible combinations of variables defined in the advanced COPD consensus. It also includes the frequencies of readmissions at 3 months and of deaths at the three recorded time points, as well as the adjusted RR for readmission or death compared with patients who did not meet the aforementioned criteria. Likewise, Fig. S1 (forest plot) shows RRs and 95% confidence intervals for 12-, 24-, and 36-month mortality based on different combinations of advanced COPD criteria.
Frequency of patients from the SocioEPOC Cohort who meet the criteria for advanced COPD, as well as the frequency of readmissions, mortality, and risk ratios in the multivariate analysis.
| Included Criteria | % in the entire sample | Readmissions in 3 months | Mortality in 12 months | Mortality in 24 months | Mortality in 36 months | ||||
|---|---|---|---|---|---|---|---|---|---|
| % | RR (CI95%)a | % | RR (CI95%)a | % | RR (CI95%)a | % | RR (CI 95%)a | ||
| FEV1<50%+Dyspnea 3–4 mMRC+CRF | 20.3 | 54.1 | 1.6 (1–2.5) | 37.8 | 2.6 (1.6–4.4) | 54.1 | 2.6 (1.6–4.3) | 61.3 | 2.3 (1.4–3.7) |
| FEV1<50%+Dyspnea 3–4 mMRC+Limitation for basic ADL | 19.8 | 55.2 | 2.2 (1.4–3.4) | 38 | 2.6 (1.2–3.5) | 53.7 | 2.2 (1.2–3.6) | 61.1 | 2 (1.2–3.2) |
| FEV1<50%+CRF+Limitation for basic ADL | 16.5 | 54.5 | 1.5 (1–2.5) | 41.1 | 2.9 (1.7–5.6) | 58.8 | 3.3 (1.9–5.6) | 64.4 | 2.5 (1.5–4.3) |
| The four criteria | 12.4 | 56.1 | 1.9 (1.1–3.2) | 44.1 | 3.4 (1.8–5.8) | 63.3 | 4.1 (2.3–7.2) | 69.1 | 3.3 (1.8–6) |
Adjusted for age, sex, BMI, number of comorbidities (heart disease, arterial hypertension, arteriopathy, diabetes mellitus, anxiety/depression, anemia, sleep apnea, malignancy), number of hospitalizations in the previous year, and CAT score.
Abbreviations: ADL: activities of daily living (feeding, dressing, bathing, toilet use, going up/down stairs and chair transferring); BMI: body mass index; CAT: COPD Assessment Test; CI95%: 95% confidence interval; CRF: chronic respiratory failure; FEV1: forced expiratory volume in the first second; mMRC: modified Medical Research Council; RR: risk ratio.
All possible combinations of variables included in the recent Spanish consensus were significantly and independently associated with readmissions and mortality at 12, 24 and 36 months. The RRs were approximately 2 for readmissions and between 2 and 4 for 12-, 24- and 36-month mortality. The likelihood of readmission or death was similar for patients with FEV1<50% and any combination of two other additional criteria (dyspnea 3–4 of the mMRC scale, CRF or limitation in basic ADL) and was only slightly higher when the patient had all 3 criteria. Previous hospitalizations were associated with both readmissions and mortality in all the analyses performed, with age and BMI also being independent predictors of death in most of the different analyses. In contrast, the CAT questionnaire score and the number of comorbidities did not show significant predictive value (the data analysis is available in Supplemental Table S1).
The present study shows that approximately one-fourth of patients admitted for COPD exacerbation meet the criteria for advanced COPD, as defined by the recent Spanish consensus. Moreover, these criteria demonstrate significant predictive capacity for medium- to long-term outcomes, highlighting that between 60 and 70% of the patients had died within three years. These patients exhibit a high consumption of health care resources, which may reflect unmet needs and/or a poor response to treatment. Their early detection and integration into palliative care models could contribute to more efficient management of health resources and better relief of both physical and emotional symptoms.
Although other variables such as previous hospitalizations, age and BMI, were also related to the risk of readmission and/or mortality, the present study did not aim to develop a predictive prognostic model, but rather to validate the usefulness of the recently agreed-upon criteria for advanced COPD established by the Spanish group [5]. In scientific literature, numerous publications have proposed predictive models for events such as readmissions or mortality (individually or combined), and the systematic reviews show a high degree of heterogeneity among them, with a moderate prognostic capacity at best [4,10]. The setting in which each study is conducted (stable phase vs. hospitalization) can be considered a key factor. These findings are applicable exclusively to patients hospitalized for severe COPD exacerbations and should not be generalized to those with stable disease. Further research is needed across different clinical settings (general/monographic consultations, day hospitals, patients with multiple chronic conditions units, etc.) to confirm the validity of these criteria.
In conclusion, the simplicity and clarity of the consensus criteria make them particularly valuable in clinical practice for identifying a subgroup of patients with a high medium- and long-term mortality rate. This subgroup includes patients who combine high symptom burden (dyspnea 3–4 on the mMRC scale), loss of autonomy, and the need for continuous support (home oxygen therapy). In these patients, severe obstruction (FEV1<50%) plays a central role, even in the presence of advanced comorbidities, highlighting the need for closer monitoring and proactive care planning.
Authors’ contributionsAll authors were involved in the conception and design of the work; acquisition, analysis, interpretation of data, drafting the work, revising it critically for important intellectual content.
Artificial intelligence involvementNothing in the study was developed with the help of any artificial intelligence software or tool.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestAFV declares research support, lecture fees and participation in advisory board in AstraZeneca, Chiesi, Grifols and GlaxoSmithKline.
AFG declares no conflicts of interest.
CRR has received honoraria in the past 3 years for lecturing, scientific consulting, clinical trial participation, or publication writing for: AstraZeneca, Boehringer Ingelheim, Chiesi, Faes farma, and GlaxoSmithKline.
RG has received speaking or advisory fees, or economic aid to attend congresses from AstraZeneca, GSK, Novartis, FAES, Chiesi, Mundipharma, Menarini, TEVA, Grifols, Ferrer, Boehringer Ingelheim, Rovi, and Gebro.
JMFG has received honoraria for speaking engagements and funding for conference attendance from Laboratories Esteve, MundiPharma, AstraZeneca, Boehringer Ingelheim, Ferrer, Menarini, Rovi, GSK, Chiesi, Novartis, and Gebro Pharma.
JMD has received grants and honoraria from AstraZeneca, BIAL, Boehringer Ingelheim, Chiesi, FAES, Gebro, GSK, Janssen, Menarini, Novartis, Sanofi, Roche, Teva, Pfizer, and Zambón.
MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Glenmark Pharmaceuticals, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols, and Novartis; consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, BEAM Therapeutics, BridgeBio, Chiesi, GlaxoSmithKline, CSL Behring, Ferrer, Inhbrix, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi/Regeneron, Zambon, Zentiva, and Grifols; and research grants from Grifols.