A disease has two different, independent, but interrelated dimensions: the clinical manifestations and the biological activity. An asthmatic is controlled if the disease does not manifest itself clinically, and asthma is in remission if there is no bronchial hyperresponsiveness (BHR) and inflammation (the two determinants of asthma pathophysiology). There may be patients who have controlled clinical manifestations but in whom disease activity persists and, conversely, there may be patients in remission, without disease activity, but with clinical manifestations (e.g. due to non-reversible bronchial obstruction). Remission is a concept imported from other inflammatory diseases (rheumatoid arthritis, inflammatory bowel disease, etc.) and it has been gaining prominence in asthma in recent years. In this way, a group of asthma experts distinguished between clinical remission (12 or more months without significant symptoms measured by an appropriate instrument, lung function optimization or stabilization, patient/provider's agreement on remission, and no use of systemic corticosteroids) and complete remission (clinical remission plus objective resolution of inflammation and, if appropriate, negative BHR).1 Remission can occur spontaneously or in response to treatment. Remission rates in childhood asthma are highly variable, depending on the population studied and the definition of remission adopted. Complete remission of asthma (defined as no symptoms, no use of inhaled corticosteroids, normal lung function, and no BHR) was found to be present in 22% of children diagnosed with allergic asthma after a mean follow-up of 30 years.2 Data in adult-onset asthma is scarcer, but complete remission is rare, especially in patients with severe disease.3
From a physician's point of view, clinical remission is no different from other terms that have already been used, such as “super-response” or “complete response”.4 However, we believe that there are compelling reasons to explore this concept further:
- 1.
The pivotal clinical trials that have led to the approval of biologic drugs by the regulatory agencies have been designed to assess the efficacy of treatment on individual outcome variables (exacerbations, need for maintenance oral corticosteroids, etc.). However, the clinicians’ perspective has shifted the focus towards a more holistic and demanding assessment of the response to biologics. In this regard, it should be noted that less than half of biologic-treated patients achieve clinical remission as defined above,5,6 highlighting the need for other therapeutic options.
- 2.
The concept of complete remission establishes additional therapeutic goals, such as inflammation and BHR, which go beyond the four clinical and functional parameters used to define clinical remission (exacerbations, use of systemic corticosteroids, symptoms and pulmonary function). BHR can persist in patients with clinical remission,7 and it has been reported that BHR is a risk factor for the development of chronic airflow obstruction.8 On the other hand, active inflammation may also be present in asthmatics in clinical remission.9 Several recently published studies showed that T2 biomarkers—blood eosinophils and fractional exhaled nitric oxide (FeNO)—have a predictive capacity for asthma attacks similar (and additive) to the independent risk seen with other well-established risk factors such as a history of an exacerbation in the prior 12 months and asthma severity graded according to the Global Initiative for Asthma (GINA) treatment steps.10–12 Moreover, the prognostic value of blood eosinophils and FeNO was additive, and this is something to be expected, as each biomarker signals different sub-pathways of the inflammatory cascade: FeNO reflects IL-4/IL-13 activity, while blood eosinophils reflect circulating IL-5 and the systemic pool of available effector cells. This has been embedded in the development of a prototype asthma attack risk scale (centred on these two biomarkers) that provides a framework for preventive, trait-based asthma management.13 However, it has yet to be demonstrated that this therapeutic strategy—which genuinely takes into account future risk—provides an objective advantage for patients while avoiding the potential risk of overtreatment.
- 3.
Focusing on complete remission could be helpful in our main objective, to modify the natural history of asthma by bringing it back to normal. The natural history of asthma results in a symptomatic burden for patients, a high risk of exacerbations, long-term loss of lung function and, in some cases, even death. All these undesirable outcomes are subject to variability in disease severity among patients. The current asthma strategy is aimed at controlling symptoms, avoiding exacerbations and, at best, normalizing lung function, but does not consider the accelerated loss of FEV1 (“steeper decline” has been defined as FEV1 loss≥54ml/yr) that is observed in a percentage of patients, which varies according to the published studies but is more frequent in patients with severe asthma.14 Given the deleterious effect on patients’ health of developing fixed bronchial obstruction, optimal treatment (e.g. a monoclonal antibody) should prevent it. As an example, the ATLAS study will establish dupilumab's role in preventing or slowing the long-term loss of lung function over three years compared with standard of care therapy, as well as its potential effect on disease modification.
- 4.
Theoretically, failure to attain an early clinical remission may reduce the chances of achieving it in the long term. A post hoc analysis of the QUEST and TRAVERSE clinical trials showed that, at week 52 of QUEST, 31.7% dupilumab-treated vs. 17.7% placebo-treated patients achieved clinical remission, whereas, at week 48 of TRAVERSE (patients were included in this study once they completed QUEST), 36.4% and 29.6% of dupilumab/dupilumab and placebo/dupilumab patients (received placebo in the first trial and dupilumab in the second) attained clinical remission.15 This could reflect a “missed opportunity”.
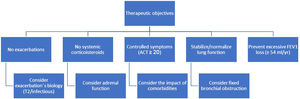
There are major obstacles in the clinical assessment of complete remission. Measurement of BHR is laborious and may be contraindicated in patients with bronchial obstruction; direct assessment of airway inflammation (bronchoscopy, induced sputum) is complex; some patients may have fixed obstruction, not amenable to improvement with treatment; and symptoms may be greatly impacted by the co-morbidities that accompany asthma. We are in the early stages of developing this concept and more evidence needs to be generated to support it. In the meantime, we believe it is reasonable to adopt a therapeutic strategy aimed at modifying the natural history of asthma (Fig. 1).
Conflicts of interestDr. Pérez de Llano reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, grants and personal fees from TEVA, personal fees and non-financial support from Novartis, personal fees and non-financial support from Chiesi, personal fees from Sanofi, personal fees from Menarini, grants and personal fees from Esteve, personal fees from MSD, personal fees from TECHDOW PHARMA, grants and non-financial support from FAES, personal fees from Leo-Pharma, personal fees from GEBRO, personal fees from GILEAD, outside the submitted work. Dr. Veiga reports personal fees and non-financial support from Chiesi, non-financial support from GSK, personal fees and non-financial support from AstraZeneca, non-financial support from Teva, non-financial support from Menarini, personal fees and non-financial support from FAES, outside the submitted work. Dr Dacal Rivas reports personal fees and non-financial support from Esteve, personal fees and non-financial support from Boehringer-Ingelheim, personal fees and non-financial support from GSK, non-financial support from Novartis, non-financial support from TEVA, non-financial support from Chiesi, non-financial support from Ferrer, personal fees from FAES Farma, outside the submitted work.