Asthma is not a homogeneous pathology. In the past it was broadly categorized into intrinsic asthma, where no obvious trigger for disease was evident, and extrinsic asthma, where a more obvious relationship to an allergen was apparent. Extrinsic asthmatics present atopy and a more striking family history of asthma than intrinsic asthmatics. Burrows et al., in 1989, demonstrated that immunoglobulin E (IgE) declined with age, but that asthmatics of all ages had higher levels of IgE than non-asthmatic subjects, and suggested that all asthma has an allergic basis.1 The terms intrinsic and extrinsic subsequently ceased to be used. However, today's enthusiasm for personalized medicine has revived interest in the concept of dissecting different forms of asthma.
Cohorts of asthma patients have revealed clusters that are distinguished on the basis of clinical characteristics that are uninformative from the point of view of the etiology of the disease. The Severe Asthma Research Program in the US identified 5 clusters based on forced expiratory volume in 1 second (FEV1), post bronchodilator FEV1, and age at onset of disease.2 The clusters were somewhat distinct, though overlapping. A study from the UK identified other clusters that included subjects with more or less sputum eosinophilia that were aligned with symptoms and clusters in which inflammation and symptoms were not concordant.3 A more recent cluster analysis based on sputum cytokines has also identified distinct patterns of asthma.4
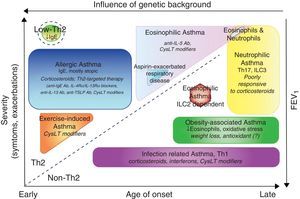
Some asthmatics are subject to frequent or severe exacerbations. Since viral infections are the principal cause of exacerbations that lead to emergency room visits, susceptibility to these events may be linked to susceptibility to epithelial infection. Defective epithelial interferon responses have been demonstrated and linked to persistence of viral replication within epithelial cells.5 Asthmatics with persistent sputum eosinophilia despite high-dose inhaled corticosteroids are a subset that have a higher relative risk of exacerbation. Efforts to phenotype asthma have increased greatly since the introduction of biologics. A phenotype is any observable characteristic of a disease without any implication of a mechanism. A variety of clinical descriptors are used to describe asthma triggered by exercise, aspirin intolerant asthma, and asthma in the obese and in the elderly (Fig. 1). Phenotypes do not necessarily carry information with regard to the underlying pathobiology of asthma, and it would be preferable to classify asthma on a pathobiological basis. Since asthma is an inflammatory disease, factors related to inflammation have been the focus of most attempts at phenotyping. As more than 150 genes have been found to carry a risk of asthma, there are likely to be many molecular phenotypes based on inherited traits alone, with overlapping characteristics. However, understanding the molecular basis for varying forms of asthma or for endotyping must remain an objective.
Clinical phenotypes of asthma and treatment. Phenotypes are represented according to the age of onset, severity of the disease, Th2 or non-Th2-dependent inflammation and influence of genetic background. In addition to classical therapy with β agonist, the specific treatments for each group are reported in italic. Ab, antibody; CysLTs, Cysteinyl leukotrienes; TSLP, thymic stromal lymphopoietin; ILC, innate lymphoid cells.
Atopy is a frequent host characteristic that is a pre-requisite for susceptibility to asthma. Sensitization and exposure to allergens as a cause of asthma is well supported by epidemiological studies, in particular those addressing occupational asthma. The immunobiology of allergic inflammation has been explored using murine models that have provided valuable insights into the basis for the inflammatory process. The identification of two CD4 T cell subsets producing different profiles of cytokines advanced the field of T cell biology. CD4 cells (Th2) producing preferentially interleukin (IL)-4 and IL-5 are important for immunoglobulin synthesis and eosinophil differentiation, survival and activation, respectively. IL-13 has additional properties such as promoting mucous cell differentiation, fibroblast and smooth muscle activation.6 Allergic inflammation is triggered in an environment which promotes CD4 Th2 differentiation, based on the influence of such factors as IL-4 itself, thymic stromal lymphopoietin (TSLP) and various eosinophil products, such as cysteinyl-leukotrienes and eosinophil derived neurotoxin.
An assessment of gene expression patterns in airway epithelial cells has shown that transcriptomes may be used to categorize subjects as having Th2-high asthma with elevated IgE, eosinophilia and responsiveness to inhaled corticosteroids, or Th2-low asthma, which is less responsive to inhaled corticosteroid.7 IL-13-driven epithelial genes, and in particular periostin, were identified. While serving as a biomarker for Th2-high asthma, periostin itself may participate in pathogenesis by promoting eosinophil recruitment to the airways, activating transforming growth factor-β to induce collagen synthesis by fibroblasts, and differentiating fibroblasts to myofibroblast.8
Currently, asthmatic patients that respond poorly to usual treatment, including high-dose inhaled corticosteroids and long acting beta agonists in combination, in some cases accompanied by oral corticosteroids, are assessed for atopy. If the skin-prick test is positive, and if serum IgE is elevated, such asthmatics often benefit from omalizumab, a humanized monoclonal antibody that binds free IgE. The treatment is most effective at reducing exacerbations, although in some patients improvements in FEV1 may also occur. Mepolizumab, an anti-IL-5, has been shown to be effective in asthmatics that have eosinophilia that is resistance to steroid treatment, and exacerbation rates are reduced in this small subset.9 Other anti-IL-5 biologics, together with an anti-IL-5 receptor antibody that might also deplete IL-5 receptor-expressing basophils, will shortly be made available. IL-13 enhances airway responsiveness and IgE synthesis in humans, and has been targeted in clinical trials with receptor antagonists (Pitrakinra) and neutralizing antibodies (Lebrikizumab).10 New alternative targets that have shown efficacy against allergen challenge in humans are TSLP and IL-33, called alarmins, which are markers of epithelial injury. These molecules activate innate lymphoid cells that may secrete an array of cytokines similar to Th2 cells that may cause the pattern of inflammation that is typical of asthma and airway hyperresponsiveness. These cells provide a non-atopic asthma pathway, and may explain some of the cases of intrinsic asthma and irritant-induced asthma.
Until recently, phenotyping of asthma may have seemed a sterile exercise. However, novel therapies have altered the therapeutic landscape. Omalizumab is effective in around 60% of patients in real-world trials, similar to clinical trials. Anti-cytokine treatments are also of proven efficacy in relatively uncommon patient populations that need careful phenotyping to ensure the expensive new therapies are correctly prescribed.
Please cite this article as: Martin JG, Panariti A. Fenotipos del asma, ¿son importantes? Arch Bronconeumol. 2017;53:177–179.