In bronchiectasis, mucociliary clearance could be disrupted by excessive production of sputum as well as for changes in the viscoelastic properties of mucus.1,2 It has been suggested that sputum rheology could be a useful differential biomarker in muco-obstructive lung diseases,3,4 wherein viscoelasticity tends to be increased.2 Particularly in bronchiectasis, rheology is altered and differs according to the aetiology,5 the severity of airway inflammation,2 just as Pseudomonas aeruginosa phenotype.1
Sputum colour chart (SCC) is a simple and quick clinical tool for bronchiectasis scoring sputum into mucoid, mucopurulent and purulent.6 Sputum colour has been associated with the number of affected lobes, bronchiectasis morphological types, lung function, health-related quality of life, need of inhaled corticosteroids,6 bacterial colonization,6,7Pseudomonas aeruginosa infection,7,8 neutrophilic airways inflammation,7 and mucus clearance,9 where the purulent one has been related with poorer outcomes. However, its relationship with sputum rheology has never been described. Therefore, the aims of this study were: (i) to analyze the association between rheological properties and sputum colour in bronchiectasis and (ii) to explore the relationship between the sputum colour and other clinical outcomes such as lung function, bronchiectasis severity, quality of life and microbiological isolation of sputum.
We conducted a cross-sectional study. The inclusion criteria were as follows: (1) bronchiectasis adults ≥18 years diagnosis confirmed by high-resolution computed tomography; (2) ability to perform all clinical tests; and (3) signed informed consent. Exclusion criteria were: (1) diagnosis of cystic fibrosis, sarcoidosis, tuberculosis or active non-tuberculosis mycobacterial infection; (2) any physical and psychological disorder that might interfere with protocol compliance; and (3) participation in any intervention study (clinical trials) in the preceding 6 months. Research Ethics Committee: HCB-0236.
Sputum samples were expectorated spontaneously (one sample per patient) and classified according to the SCC.6 Samples were divided in two parts: one for microbiological analysis and the other for rheological characterization. For rheological analysis a HAAKE RheoStress 1 rheometer (ThermoFisher, MA) with 35mm serrated parallel plate geometry was used. Samples were loaded onto a Peltier plate set at 37°C and with a distance between plates of 400μm. An oscillatory test was done for shear stress (τ), ranging from 0.1Pa to 5Pa at an angular frequency (ω) of 1rad/s. The storage modulus (G′), loss modulus (G″), complex viscosity (|η*|) and strain (γ) were recorded. Casson yield stress (ko), and Casson plastic viscosity (kμ) were computed by numerically adjusting the recorded data to the Casson model.10 Other clinical outcomes were collected: chronic therapy, quality of life using QoL-B questionnaire,11 severity of bronchiectasis using Bronchiectasis Severity Index (BSI)12 and FACED score13 and lung function with EasyOne™ World Spirometer (ndd Medical Technologies, Zurich, Switzerland).
Descriptive statistics were used for basic features of study data, and appropriate statistical tests were performed to compare groups. Categorical variables were compared via Fisher's exact test, while continuous variables in the three groups were contrasted by the analysis of variance (ANOVA) or the non-parametric Kruskal–Wallis test with post hoc pairwise comparisons using Bonferroni's adjustment to control the family-wise error rate.
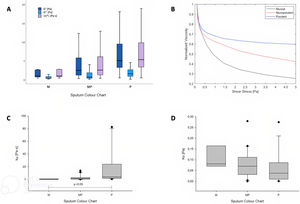
Sixty-four samples were included and classified as: mucoid (11%), mucopurulent (56%) and purulent (33%) (Table 1). Purulent samples had higher values of G′, G″ and |η*| at τ=1Pa compared to mucopurulent and mucoid ones (p<0.05) (Fig. 1A), and lower values of γ at τ=1Pa (purulent γ 0.2[0.1–0.3] vs. mucopurulent 0.4[0.2–0.8] vs. mucoid 1[0.4–1.3], p<0.01)). Normalized viscosity of sputum showed a non-Newtonian shear-thinning fluid behaviour when increasing τ independently of colour (Fig. 1B). In addition, the evolution of viscosity showed higher values according to the sputum colour and these were maintained independently to the shear stress (Fig. 1B). The Casson plastic viscosity (Fig. 1C) showed a statistically significant difference between purulent and the other groups (p<0.05). On the other hand, the Casson yield stress (Fig. 1D) showed no differences between groups, which is an indicator that the fluid behaviour is the same in all groups. Moreover, patients with purulent colour had worse levels of quality of life compared to mucopurulent group, specifically in the domains of role function, vitality, treatment burden and health perceptions. On the other hand, bronchiectasis severity according to FACED score was statistically different between groups (p=0.033), where light was associated with higher mucopurulent colour, moderate to mucoid, mucopurulent and purulent ones and finally severe to mucopurulent and purulent groups.
Descriptive characteristics according to sputum colour.
| All patients | Mucoid | Mucopurulent | Purulent | p value | |
|---|---|---|---|---|---|
| N=64 | 7 (11) | 36 (56) | 21 (33) | ||
| Sex, female | 26 (40.6) | 2 (28.6) | 12 (33.3) | 12 (57.1) | 0.166 |
| BSI scores | 0.393 | ||||
| Light | 5 (7.8) | 0 (0) | 4 (11.1) | 1 (4.7) | |
| Moderate | 12 (18.7) | 1 (14.3) | 9 (25) | 2 (9.5) | |
| Severe | 47 (73.4) | 6 (85.7) | 23 (63.9) | 18 (85.7) | |
| FACED score | 0.033 | ||||
| Light | 8 (12.5) | 0 (0) | 7 (19.4) | 1 (4.7) | |
| Moderate | 37 (57.8) | 7 (100) | 20 (55.5) | 10 (47.6) | |
| Severe | 19 (29.6) | 0 (0) | 9 (25) | 10 (47.6) | |
| Microbiological culture | 0.053 | ||||
| Mucoid Pseudomonas aeruginosa | 30 (47) | 0 (0) | 19 (53) | 11 (52.3) | |
| Non-mucoid Pseudomonas aeruginosa | 13 (20.3) | 4 (57) | 7 (19.4) | 2 (9.5) | |
| No organism reported | 14 (21.8) | 3 (43) | 6 (16.7) | 5 (23.8) | |
| Other | 7 (11) | 0 (0) | 4 (11.1) | 3 (14.3) | |
| Rheology characteristics at τ=1Pa | |||||
| G′ (Pa) | 3.15 [1.6–6.6] | 0.96 [0.52–2.74] | 2.5 [1.2–5.8] | 5.1 [3.14–13.8] | 0.008 |
| G″ (Pa) | 0.94 [0.46–1.92] | 0.38 [0.32–1.1] | 0.69 [0.35–1.9] | 1.6 [0.87–3.6] | 0.019 |
| |η*|[Pas] | 3.24 [1.7–6.9] | 1.01 [0.64–2.8] | 2.6 [1.3–6.12] | 5.34 [3.23–14.4] | 0.009 |
| γ [–] | 0.31 [0.14–0.6] | 1 [0.36–1.6] | 0.39 [0.16–0.8] | 0.19 [0.07–0.31] | 0.009 |
| Quality-of-Life Bronchiectasis Questionnaire | |||||
| Physical function | 45.8 (31.9) | 53.3 (38) | 50 (34.6) | 37.04 (25.3) | 0.160 |
| Role function | 59.5 (32.8) | 73.3 (43.4) | 69.1 (25.5) | 40.7 (33.4) | 0.026a |
| Vitality | 54.3 (30.5) | 66.7 (40.8) | 63.1 (22.8) | 37.04 (32.1) | 0.015a |
| Emotional function | 73.2 (29.1) | 86.7 (29.8) | 80.9 (23) | 57.4 (31.9) | 0.050 |
| Social function | 49.7 (37.6) | 66.7 (47.1) | 52.4 (36.2) | 40.7 (37.1) | 0.499 |
| Treatment burden | 74.5 (27.9) | 66.7 (40.8) | 82.14 (21.2) | 64.8 (31.2) | 0.040a |
| Health perceptions | 33.3 (23.3) | 33.3 (31.2) | 41.7 (21.9) | 20.4 (17.7) | 0.019a |
| Respiratory symptoms | 63.1 (27.3) | 80 (29.8) | 65.5 (26.4) | 54.6 (26.7) | 0.080 |
Abbreviations: BSI, bronchiectasis severity index.
Data are presented as n (%), mean (SD) or median (p25–p75). p-Values in bold are statistically significant.
In this study, we have described for the first time an association between the SCC and the viscoelastic characteristics of sputum in bronchiectasis. Although rheology has been suggested as a useful differential biomarker in muco-obstructive lung diseases,3,4 the need of an especial equipment and technical formation may difficult its implementation; so, it is therefore key to find strategies to correlate rheology measures to easier clinical assessments. The results of our study are on this line because we have found an association between viscoelastic properties and the SCC, which is a fast, cheap, and validated tool that have already been included in the daily clinical practice.6 SCC has been described as a useful and easily biomarker that could detect the presence of inflammatory cells in the airway,14 and now could be also associated to the rheology of the sputum. Our findings hypothesize that patients with purulent colour, which have higher viscoelastic values, may benefit from physiotherapy strategies to fluidize sputum,15 but further research is needed.
Regarding rheological data, in this study we have shown that viscosity decreased with the stress applied, so it is not a valid parameter for comparison between trials if no other parameters are defined. Thus, Casson yield stress and plastic viscosity are stronger parameters since they are independent of shear stress and frequency in the rheological experiments.
The study has the limitation that the number of mucoid samples is very low. However, we highlight the strength that we have also analyzed viscoelastic properties in a dynamic way, helping us to understand how it could change at different shear stress values. We found that the viscoelastic behaviour did not change throughout the experiment, fortifying the results of our study. On the other hand, the association between SCC and viscoelastic properties was independently of other clinical outcomes such as chronic therapy, microbiological analysis, severity of the disease or lung function. Our results showed a difference regarding quality of life where the purulent group had worse scores in comparison with the other two groups. This finding could be related to symptom's burden, hypothesizing that this group could have more difficulties in airway clearance and therefore directly affecting health related quality of life.16 Finally, sputum colour was also associated with bronchiectasis severity according FACED score, where severe patients had higher number of mucopurulent and purulent ones.
In conclusion, bronchiectasis samples classified as purulent showed increased viscoelastic values compared to mucoid and mucopurulent ones, thus impairing mucociliary clearance. These results were maintained independently of time, applied effort, bronchiectasis severity, microbiological isolation as well as the baseline treatment.
FundingInstituto de Salud Carlos III (FIS PI1800145). ISCIII-FEDER, ICREA Academia, SEPAR grants 208 and 628. The funding sources were not involved in study design, collection, analysis, and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Conflict of interestsThe authors state that they have no conflict of interests.