Acute respiratory distress syndrome (ARDS) is a frequent and life-threatening entity. Recently, it has been demonstrated that diffuse alveolar damage (DAD), which is considered the histological hallmark in spite of presenting itself in only half of living patients with ARDS, exerts a relevant effect in the ARDS outcome. Despite the fact that the bronchial tree constitutes approximately 1% of the lung volume, discovering a relation between DAD and bronchial tree findings could be of paramount importance for a few reasons; (a) it could improve the description of ARDS with DAD as a clinical–pathological entity, (b) it could subrogate DAD findings with the advantage of their more accessible and safer analysis and (c) it could allow the discovery of new therapeutic targets. This narrative review is focused on pathological airway changes associated to Diffuse Alveolar Damage in the context of Acute Respiratory Distress Syndrome. It is organized into five sections: main anatomical and functional features of the human airway, why it is necessary to study airway features associated to DAD in patients with ARDS, pathological airway changes associated with DAD in animal models of ARDS, pathological airway changes associated with DAD in patients with ARDS, and the newest techniques for studying the histology of the bronchial tree and lung parenchyma.
El síndrome de distrés respiratorio agudo (SDRA) es una patología frecuente con riesgo asociado para la vida de los pacientes. Recientemente, se ha demostrado que el daño alveolar difuso (DAD), considerado como la manifestación histológica característica del síndrome, además de presentarse en la mitad de los pacientes que padecen SDRA, posee un papel relevante en la evolución de este. A pesar del hecho de que el árbol bronquial constituye aproximadamente un 1% del volumen pulmonar, el descubrimiento de una asociación entre el DAD y hallazgos en el árbol bronquial podría ser muy importante por algunas razones: (a) podría mejorar el diagnóstico del SDRA con DAD como entidad clínico-patológica, (b) podría subrogar marcadores de DAD, cuyo análisis sea más seguro y accesible, (c) podría favorecer el descubrimiento de nuevas dianas terapéuticas. Esta revisión narrativa se centra en las alteraciones patológicas de las vías respiratorias asociadas al daño alveolar difuso en el contexto del síndrome de distrés respiratorio agudo. El texto se compone de cinco secciones: principales características anatómicas y funcionales de las vías respiratorias en humanos, por qué es necesario estudiar las características de las vías respiratorias asociadas al daño alveolar difuso en los pacientes con síndrome de distrés respiratorio agudo, alteraciones patológicas de las vías respiratorias asociadas con DAD en modelos animales de SDRA, alteraciones patológicas de las vías respiratorias asociadas con DAD en pacientes con SDRA y nuevas técnicas para el estudio histológico del árbol bronquial y el parénquima pulmonar.
Acute respiratory distress syndrome (ARDS) is a frequent and cataclysmic entity with a worldwide mortality of 40%.1 The recent demonstration that diffuse alveolar damage (DAD) exerts a relevant influence on ARDS outcome reinforces the postulation that DAD is the histological hallmark of ARDS.2–6 Furthermore, the latest recognition that only half of living patients with ARDS present DAD suggests that a great bulk of the knowledge generated from ARDS studies,4–7 which did not distinguish between patients with and without DAD, should be reinterpreted.8–10 This may also be one of the main reasons to explain the lack of effective pharmacological therapies for this syndrome.2,9–13
The lung anatomy has been typically organized into two sectors, the lung parenchyma, and the bronchial tree. Numerous studies have focused their attention on lung parenchymal changes associated with ARDS5–7,14–18 but very few have focused on the airways. Indeed, as far as we know, all clinical studies focused on airways changes associated with ARDS should be considered cautiously because they do not consider the histology.19–21 Identifying airway changes associated with DAD in patients with ARDS is not merely an academic exercise as this could modify the current perspective of ARDS and collaborate in the discovery of new therapeutic targets (vide infra).
Cryo-transbronchial lung biopsy (CTLB) is a relatively recent and less invasive technique in comparison to the traditional open lung biopsy. Given this new context of ARDS and DAD as a specific clinical pathological entity and the risk associated with traditional open lung biopsy,2,7,8,14,22 CTLB could be considered a useful tool to expand the study of the airway and lung parenchyma in high risk patients as in those with ARDS.
To the best of our knowledge, this is the first narrative review that specifically focuses on airway pathological changes associated with ARDS with DAD or lung parenchymal findings compatible with DAD. This narrative review is organized in five sections: main anatomical and functional features of the human airway, why it is necessary to study airway features associated with diffuse alveolar damage in patients with acute respiratory distress syndrome, pathological airway changes associated with DAD in animal models of ARDS, pathological airway changes associated with DAD in patients with ARDS, and the newest techniques for studying the histology of the bronchial tree and lung parenchyma. Studies that reported physiological and pathological features in the airways from animal models of ARDS or humans with ARDS but did not report DAD or lung parenchymal findings compatible with DAD as a subgroup were not considered. With the aim to facilitate the interpretation by readers, the control group of each study was explicitly mentioned.
Main anatomical and functional features of the human airwayThe bronchial tree consists of a complex series of branching tubes which have the aim of transferring gas from the atmosphere to the alveolus and vice versa. In humans, it has approximately 21–23 generations and occupies roughly 1% of the lung volume.23,24 The human airway model of Mandelbrot demonstrated that from large bronchus to peripheral bronchioles, the branching pattern is almost the same: each airway is divided into two “daughter” branches whose individual diameter is reduced by a constant factor of 0.79–0.85.25–27 According to the inner diameter, the bronchial tree is classified into two zones: large airway (diameter ≥2mm) and small or peripheral airway (diameter <2mm). This complex structure determines two main consequences23: (a) with each branching, the cross-sectional area of the airway system increases and the airway resistance decreases, and (b) the alveolus ventilation is homogenized because roughly all of the alveolus are a similar distance from the trachea.
From a functional perspective, approximately the first 13–15 generations of the airways are considered conduction airways because they do not participate in the gas exchange (anatomic dead space). The following 5–8 generations are denominated acinar airways and are characterized by the gradual incorporation of respiratory units.23
Three key aspects characterize the bronchial tree. Firstly, most of the protective mechanisms present in the large airways (e.g., neural influence, structural support and smooth muscle tone) are not present in the small airways.24 Secondly, the presence of Kohn pores allows for the maintenance of a certain degree of alveolar ventilation even when the airway providing the air to it, is occluded. Thirdly, given that airways are embedded in the connective tissue network of the lung, they have a significant interdependence with parenchyma and vascular structures.28,29 An outstanding review focused on airway–parenchymal interdependence was published by Pare et al.29
Why is it necessary to study airway features associated to diffuse alveolar damage in patients with acute respiratory distress syndrome?Four facts support the importance of studying airways changes associated with ARDS and DAD. Firstly, it was demonstrated that ARDS with DAD constitutes a specific clinical–pathological entity, but all of these studies were based only on lung parenchymal changes.5,14,17 In the event that airway features associated with DAD exist, these would be included as part of the aforementioned clinical–pathological entity (ARDS with DAD). In addition, this could also be considered another reason to include the histology as diagnostic criteria for ARDS.9,22,30
Secondly, given that the diagnoses of DAD require the analysis of lung samples usually obtained by risky procedures such as open lung biopsy, only a few centers can diagnose it.31–33 If the relation between airway features and DAD exists, the former could surrogate the latter. This is highly relevant as this could resolve the problem of DAD diagnosis and could be used as a more accessible histological pattern for developing new biomarkers and therapeutic targets (vide infra).
Thirdly, several well-designed studies demonstrated the existence of ARDS endophenotypes with different outcomes and responses to specific treatments.34,35 The latent variable that explains this clusterization is unknown, but we speculate that it could be airways and/or parenchymal features (Fig. 1).
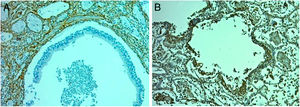
Terminal bronchiole with evidence of epithelial denudation. A – Hematoxylin and eosin (×10). Terminal bronchiole with an adjacent vessel in normal lung. Santa Clara Hospital Bogota Colombia. B – Fibronectin immunocytochemical staining (×40). Terminal bronchiole with evidence of denudation of the epithelium. Santa Clara Hospital Bogota Colombia. C – Collagen III Immunohistochemistry Staining (×10). Terminal bronchiole with evidence of denudation of the epithelium. Santa Clara Hospital Bogota Colombia.
Fourthly, in the current world of personalized medicine, it is well accepted that “one size does not fit all.” For that reason, it is essential to look for specific targets or a pathway to lump patients according to their presence and try out specific treatments on each subgroup.2,36 The airway tree could have molecules, structures or pathways that could potentially be used as a therapeutic target, in the event of being associated with DAD.
Airway changes associated with DAD in animal models of ARDSIn the early 1980s, Lachmann et al.37 described the histological changes in a guinea-pig model of ARDS (Table 1). They compared lung pathological changes before and after 8h of mechanical ventilation with pressure control mode and repeated lung lavage. In almost all animals, hyaline membranes were observed accompanied by desquamation of bronchial epithelium and peribronchial inflammatory changes characterized by edema and the infiltration of polymorphonuclear leukocytes and eosinophils.
Airways findings in animal models of acute respiratory distress syndrome.
| Author | Animal | Mechanism to induce the lung injury | Main airways finding in the non DAD group | Main airways finding in the DAD group |
|---|---|---|---|---|
| Lachmann et al.37 | Guinea-pig | Repeated lung lavage+mechanical ventilation | Not correspond | Bronchial epithelium and peribronchial inflammatory changes characterized by edema and infiltration of polymorphonuclear leukocytes and eosinophils |
| Taskar et al.38 | Rabbit | Surfactant dysfunction+mechanical ventilation | – PEEP+0% DOSS, PEEP+2% DOSS, and NEEP+0% DOSS: normal lungs (all animals) – PEEP+5% DOSS (3 of 6 animals): mild focal alveolar collapse, and one of these animals also showed mild focal intraalveolar edema | NEEP+2% DOSS group (4 of 6 animals) and NEEP+5% DOSS (all animals): alveolar collapse, intraalveolar edema, hyaline membranes, intraalveolar hemorrhage, bronchiolar epithelial necrosis, and recruitment of granulocytes to the air space |
| Tsuchida et al.39 | Rat | Repeated lung lavage+mechanical ventilation | Noninjurious ventilation: minimal alveolar and airway injury | Injurious ventilation: peribronchial infiltration of inflammatory cells, higher expression of IL6 and IL8. Severe alveolar and airway injury |
| D́Angelo et al.40 | Rat | Mechanical ventilation | Control group: – bronchiolar injury score: 6.8 (6–9) – percentage of ruptured bronchiolar–alveolar attachments: 8.9 (5–10) – distance between normal bronchiolar–alveolar attachments: 52 (47–59) | Zero end-expiratory pressure – bronchiolar injury score: 30.9 (27–33) – percentage of ruptured bronchiolar-alveolar attachments: 31.1 (23–40) – distance between normal bronchiolar-alveolar attachments: 73 (64–86) Negative end-expiratory pressure – bronchiolar injury score: 42.9 (33–52) – percentage of ruptured bronchiolar-alveolar attachments: 38.8 (29–43) – distance between normal bronchiolar-alveolar attachments: 83 (68–89) Zero end-expiratory pressure after dioctylsodium sulfosuccinate administration – bronchiolar injury score: 42.4 (32–57) – percentage of ruptured bronchiolar-alveolar attachments: 34.5 (24–41) – distance between normal bronchiolar-alveolar attachments: 77 (58–88) Ventilation with large tidal volume on positive end expiratory pressure only: – bronchiolar injury score: 9.0 (8–14) – percentage of ruptured bronchiolar-alveolar attachments: 19.7 (15–23) – distance between normal bronchiolar-alveolar attachments: 62 (47–68) |
Years before, Taskar et al.38 hypothesized that repeated alveolar collapse and re-expansion associated with surfactant dysfunction lead to lung damage (Table 1). They used a two-hit rabbit model; firstly all the animals were allocated to one of the following groups: 0, 2, or 5% dioctyl sodium sulfosuccinate (DOSS) followed by an aerosol of 99mTc-diethylene triamine pentaacetic acid (99mTc-DTPA) with the aim to induce surfactant dysfunction. Secondly, half of each subgroup was also randomized to mechanical ventilation for 3h with a positive end-expiratory pressure (PEEP) of 2cm H2O or with a negative PEEP (NEEP) of 3cm H2O with the aim to consolidate the lung injury. Three grades of pathological changes were found: (a) absence (PEEP+0% DOSS, PEEP+2% DOSS, and NEEP+0% DOSS), (b) mild (NEEP+2% and PEEP+5%) and (c) severe (NEEP+5% DOSS). The aforementioned pathological changes consist of perivascular and intra-alveolar edema, irregular areas of alveolar collapse and ductal overdistension, inflammatory cells in alveolar septa and subtle hyaline membranes in alveoli. In reference to airway affectation, 5 of the 6 animals in the group of NEEP+5% DOSS and 3 of the 6 in the group of NEEP+2% DOSS presented a certain degree of epithelial desquamation with prominent necrosis of the bronchiolar epithelium. The other groups did not present histological airway modifications.
In line with previous studies, Tsuchida et al.39 used a rat model of ARDS induced by repetitive bronchoalveolar lavage followed by 1.5h of non-injurious or injurious mechanical ventilation to study airway and lung parenchyma changes (Table 1). Controls or sham animals were not included. They reported that the alveolar and airway-associated injury was minimal after noninjurious ventilation. However, there was a marked alveolar airway epithelial injury in both dependent and nondependent regions after injurious ventilation (increased peribronchial infiltration of inflammatory cells and higher expression of interleukin 6 and 8).
Finally, D́ Angelo et al.40 used a rat model of mechanical ventilation to study airway histology changes (Table 1). All rats underwent an initial (PEEP1) and final 30-min period (PEEP2) of baseline ventilation with PEEP, separated by 2.0–2.5h. During this period, the following ventilation types were used: (a) baseline ventilation with zero end-expiratory pressure (ZEEP), (b) baseline ventilation with negative PEEP of −3cmH2O; (c) baseline ventilation with ZEEP after the surfactant function was altered with 10% alcoholic solution of dioctyl sodium sulfosuccinate; (d) high volume (Vt=26ml/kg) ventilation with PEEP and (e) baseline ventilation with PEEP (control group). Lung parenchymal injury was semi-quantitatively evaluated with a four-grade scale (0 absent and 3 marked) that included four parameters: focal alveolar collapse, perivascular and/or alveolar edema, recruitment of granulocytes to the air spaces and hemorrhage. They did not report the presence of hyaline membranes. However, they observed results compatible with DAD. The global sum of points in the (e) group was 0; in the (a) group was 1; in the (b) group was 8; in the (c) group was 29, and in the (d) group was 14. Meanwhile, the bronchial injury was 6.8 (mean) in the (e) group and in all except for the (d) group the punctuation was greater (group a: 30.9, group b: 42.9 and group c: 42.4). Likewise, the airway-parenchymal uncoupling (% [abnormal bronchiolar- alveolar attachments]) was also greater in all the groups (group b: 31%; group b: 39%; group c 35% and group d 19.7%) in comparison to the control group (9%) Finally, the distance between normal bronchiolar and alveolar attachments was also longer in all except for the (d) group (group a: 73μm, group b: 83μm and group c: 77μm) in comparison to the d group (median 52μm).
Airway changes associated with DAD in patients with ARDSMorales et al.41 compared small airway structural and inflammatory changes in two cohorts of autopsies; one included ventilated patients with ARDS (pa02/Fi02 ≤200) with DAD (n=31). The other, were controls and included non-ventilated, non-smoking patients who died of non-pulmonary causes without previous lung diseases (n=11). The interval between an ARDS diagnosis and death ranged from 1 to 24 days (16 patients died within the first 48h and 22 within the first week). In patients with and without ARDS, they analyzed the perimeter of the airway basement membrane and found that there was a significant difference in the percentage of normal (32.9±27.2% vs. 76.7±32.7%; p<0.01, respectively), abnormal (14.4±14.8% vs. 1.37±3.20%; p=0.01 respectively) and denudated epithelium (52.6±35.2% vs. 21.8±32.1%; p=0.015, respectively). Expressed as area (μm2) corrected by the basement membrane perimeter (μm), they also found that both groups differ in the thickness of the total airway wall (138.7±54.3 vs 86.4±33.3; p=0.005, respectively), the inner layer (35.2±32.0 vs 17.2±8.14; p=0.034, respectively) and the outer layer (88.7±29.9 vs 50.4±17.7; p<0.01, respectively) were different. An interesting finding is that the extension of normal epithelium was positively correlated with PaO2/FiO2 ratio (r2=0.34; p=0.02) and negatively with plateau pressure (r2=0.27; p=0.04). Likewise, the extension of denuded epithelium showed a negative correlation with the PaO2/FiO2 ratio (r2=0.27; p=.04). They also found: (a) a higher degree of airway inflammation, (b) higher content of collagen type I, fibronectin and versican in the outer airway layer, (c) higher versican expression in the inner airway layer and (d) increased expression of matrix metallopeptidase 9 in ARDS patients than in controls respectively. Furthermore, Pires-Neto et al.42 found in a subgroup of previously published autopsies41 that the expression of interleukin (IL) 8 in the airway epithelium (152.7±162.2μm2/μm vs. 25.2±59.0μm2/μm, p≤0.01), the number IL8+ (12.7±10.7cells*10−3/μm vs. 1.6±1.8cells*10−3/μm, p<0.01), the number IL6+ (25.5±14.7cells*10−3/μm vs. 13.2±16.7cells*10−3/μm, p=0.01) and the number of TUNEL-positive epithelial cells (4.5±4.6cells*104/μm vs 8.6±5.2cells*104/μm, p=0.06) were different in ARDS patients than in controls, respectively. Furthermore, they found several correlations between clinical and laboratory parameters: PaO2/FiO2 and airway wall of IL8+cells (r=−0.56; p=0.02); days of ARDS evolution and airway epithelium Fas expression (r=0.46, p=.03) and days of ARDS evolution and airway wall IL6+cell (r=−0.64, p<0.01).
The newest techniques for studying the histology of the bronchial tree and lung parenchymaWithin the array of available procedures and techniques to study the airways, we focus our attention on cryo-transbronchial lung biopsy technique (CTLB) as their special features may determine a qualitative change in the airway study.43,44 The cryosurgical equipment operates quickly with the Joule–Thompson effect, which describes the temperature change of a real gas or liquid when it is forced through a narrow area (e.g., valve or porous plug) while kept insulated so that no heat is exchanged with the environment. The cooling agent is applied under high pressure (45bar) through the central canal of the probe. The gas at the tip, usually CO2, expands due to the sudden difference in pressure relative to the atmospheric pressure, resulting in a drop in temperature at the tip of the probe. The cryoprobe is introduced into the selected area under fluoroscopic guidance via a flexible bronchoscope. A distance of approximately 10–20mm from the thoracic wall and a perpendicular relation between the thoracic wall and the probe are considered optimal. Once brought into position, the probe is cooled for approximately 3–8s. The frozen tissue attached to the probe's tip is removed by pulling the cryoprobe together with the bronchoscope. The number of biopsies taken is usually 3–6.45
The safety and diagnostic yield of CTLB was initially evaluated by Casoni et al.46 in a cohort of 69 patients with clinical and radiologic features of fibrotic diffuse parenchymal lung diseases. They reported that major complications were: prolonged bleeding (n=1); pneumothorax (n=19) and death (n=1). In addition, they mentioned that adequate cryobiopsies were obtained in 68 cases with a median size of 43.11mm2 (range, 11.94–76.25mm2) as well as the pathologists were confident that histopathologic criteria sufficient to define a specific pattern in 52 patients. More recently, Ussavarungsi et al.47 reported a cohort of 74 patients with CTBL. The mean maximal diameter of the samples was 9.2mm (range, 2–20mm) and the diagnostic was performed in 38 specimens. After the procedure, bleeding and pneumothorax occurred in 1 and in 16 patients respectively. In line with the previous study, Kronborg-White et al.48 in a cohort of 38 patients underwent CTLB reported that diagnosis was achieved in 28 with few complications (pneumothorax n=10 and local bleeding n=6).
Several studies have compared CTLB with traditional techniques. For example, Pajares et al.,49 conducted an RCT with the aim to compare the diagnostic yield and complication rate of CTLB versus conventional forceps in patients with clinical and radiographic findings of interstitial lung disease (ILD). They included 77 patients (CTLB 39 patients and conventional force 38 patients). They reported that mean duration of procedures (CTLB 30.5±7.6min vs. conventional forceps 32.5±8.6min; p=0.294), bleeding (CTLB n=39 vs. conventional forceps n=38, p=0.068) and pneumothorax (CTLB n=2 vs. conventional forceps n=3, p=0.999) were similar in both groups. However, the diagnostic yield rate was higher in the CTLB groups (5.4% vs. 29.1%, p=0.038). Similarly, Ravaglia et al.50 conducted a meta-analysis that compared the performance of CTLB (n=297) and the video-assisted thoracoscopy surgery (VATS) (n=150) in patients with interstitial lung diseases respectively. They found that CTLB was associated with a greater proportion of non-diagnostic patterns (51 vs 2, p=0.01) but the mortality rate due to adverse effect (1 vs 4, p=0.04) and the median time of hospitalization (2.6 days vs 6.1 days, p<0.01) were better. Regarding complications, in the group of CTLB, the most frequent complications were pneumothorax (n=60), transient respiratory failure (n=2) and seizures (n=2); meanwhile, in the VATS groups, the most frequent complications were persistent fever (n=7), prolonged air leak (n=5) and acute exacerbation of idiopathic pulmonary fibrosis (n=5). Dhooria et al.51 performed a meta-analysis with the aim of comparing the performance of CTLB and flexible forceps biopsy (FFB) in patients with diseases that diffusely affect the lungs. The comparison demonstrates that retrieved lung specimen (20.4mm2 [n=805] vs 4.3mm2 [n=378], p=<0.05) and proportion of positive diagnosis (139/161 vs 88/160, p<0.05) were better in the former group than in the latter group. Furthermore, major complications in the CTLB group were registered in 55 from 764 patients and correspond to pneumothorax (n=52), severe bleeding (n=2) and death (n=1).
Although the emerging data on CTLB is encouraging, we have to accept that current studies comparing CTLB and surgical procedures have some flaws: low number of patients, no focus on high-risk patients such as those with ARDS and the influence of the physician who performs the procedure may affect the complication rate.52
Another approach to studying the airway is using biomarkers. This type of molecule has been successfully used for understanding the ARDS pathogenesis, increasing the accuracy of the risk stratification once ARDS is diagnosed, targeting subgroups of patients that could respond to specific interventions or treatments and for identifying new therapeutic targets.34,35,53–61 Regarding the airways, Determann et al.62 found that baseline plasma concentration of Clara cell protein (CC16) is more expressed in patients on mechanical ventilation with ARDS than in those without ARDS (14.3 [9.0–19.0] ng/ml vs. 7.7 [4.8–13.5]ng/ml, p=0.03). In line with the previous study, Maile et al.63 analyzed a cohort of intubated patients with demonstrated or suspected inhalation injury. They found that IL10 (74 [RIQ 33; 94] pg/ml vs 45 [RIQ 28; 75] pg/ml, p=0.03), HA (390 [RIQ 150; 467] ng/ml vs 217 [RIQ 57; 389], p=.04] and dsDNA (2382 [RIQ 820; 7.396] ng/ml vs 184 [RIQ 0.0; 3054] ng/ml p=.02) in brocho-alveolar lavage obtained during the first 72h from ICU admission were associated to an increased risk of lung infection. On the other hand, an attractive approach based on biomarkers presented on exhaled breath condensate (EBC) was proposed by Roca et al.64 The hypothesis that particles exhaled in breath reflect the composition of the alveolar lining fluid for that reason the composition of EBC is influenced by lung diseases and may be modulated by therapeutic interventions. In a pilot study that included 6 mechanically ventilated adult patients with ARDS they reported relevant changes in ECB before and after salbutamol administration (pH after deaeration; conductivity; leukotriene B4; nitrite+nitrate and 8-isoprostane). In a second research from the same group,65 they used the EBC technique in 10 critically ill (mechanically ventilated patients during the weaning phase and without lung diseases) and in 20 healthy controls (non-mechanically ventilated and nonsmoker). They also reported several differences between ventilated and non-ventilated (post-deaeration pH; NO2/NO3 and 8-isoprostane). Both studies demonstrated that EBC could be a useful technique to track physiopathology lung modifications.
Recently, new techniques such as the heat moisture exchanger (HME) filter (an in-line disposable hygroscopic bacteriostatic sponge placed between the patient and the ventilator) demonstrated their usefulness to collect liquid from the airway that subsequent is analyzed. It has been hypothesized that this sample represents changes in distal airspace and the utility in the diagnosis of ventilator-associated pneumonia 66,67 or in the differentiation between ARDS and hydrostatic edema has been demonstrated.68
Unfortunately, to the best of our knowledge, no molecule has been validated using the human airway histology as the gold standard. For that reason, none of these biomarkers can surrogate the histology airways findings in patients with ARDS.
ConclusionSince recent evidence to support that DAD is the histological gold pattern of ARDS, the relevance of its diagnosis increases dramatically. Although some animal and human evidence argue in favor of an existing relationship between DAD and airway findings, it is clear that the knowledge is scarce, confusing and difficult to interpret. The main handicap of the knowledge related to airway findings associated with DAD in patients with ARDS is the lack of studies that report airway findings in patients or autopsies with ARDS but without DAD. Otherwise, since diagnosing DAD in the real world is complex and risky, identifying airway histological patterns associated with DAD may provide a great opportunity to use these findings instead of DAD. Given that CTLB is a less invasive procedure than surgery, it could be useful as a technique that allows diagnosing the pathology pattern and discovering new surrogating biomarkers in patients with ARDS. Unfortunately, at this moment no studies have applied CTLB in patients with ARDS.
Conflict of interestThe authors declare no conflict of interest.