Yin Yang 1 (YY1) is a transcriptional repressor that inhibits muscle gene expression and myogenesis. YY1 has not previously been investigated in the skeletal muscle of patients with COPD. The aims of this study were to investigate YY1 expression and localisation in the quadriceps muscle of COPD patients compared to healthy age-matched controls, and to examine the relationship between YY1 expression and localisation and quadriceps muscle fibre cross-sectional area (CSA) in COPD patients.
Patients and methods15 COPD patients and 8 age-matched controls underwent lung and quadriceps function assessments and a percutaneous quadriceps biopsy. Quadriceps muscle fibre CSA and fibre proportions and YY1 localisation were determined by immunofluorescence. YY1 was immunoprecipitated from muscle and YY1 levels assessed by western blotting.
ResultsYY1 levels were inversely correlated with type IIx and type I fibre CSA in patients and controls, though YY1 levels were not significantly different between the groups. Nuclear localisation of YY1 was demonstrated in the patients but not in controls.
ConclusionYY1 expression is associated with smaller quadriceps fibre CSA in COPD and nuclear localisation of YY1 was found in muscle of patients but not controls. Regulation of YY1 appears altered in COPD and may be implicated in COPD-related muscle atrophy.
El Ying Yang 1 (YY1) es un factor de transcripción represor que inhibe la expresión génica muscular y la miogénesis. Este factor no se ha investigado previamente este factor no se ha investigado en el músculo esquelético de pacientes con enfermedad pulmonar obstructiva crónica (EPOC). Los objetivos del presente estudio fueron investigar la expresión de YY1 y su localización en el músculo cuádriceps de pacientes con EPOC, comparado con individuos control sanos, emparejados por edad, y examinar la relación entre la expresión y localización de YY1 en las áreas transversales (AT) de las fibras musculares del cuádriceps en pacientes con EPOC.
Pacientes y métodosSe sometió a 15 pacientes con EPOC y a 8 individuos de control, emparejados por edad, a valoraciones de la función pulmonar y del cuádriceps y a una biopsia percutánea de este músculo. Mediante inmunofluorescencia se determinó el AT de las fibras musculares del cuádriceps las proporciones de fibras y localización de YY1. YY1 se inmunoprecipitó a partir del músculo y sus niveles se evaluaron mediante inmunotransferencia.
ResultadosLos niveles de YY1 se correlacionaron inversamente con el AT de las fibras de tipo IIx y de tipo I en pacientes e individuos de control, aunque los niveles de YY1 no fueron significativamente diferentes entre ambos grupos. En los pacientes, pero no en los individuos control, se demostró la localización nuclear de YY1.
ConclusiónLa expresión de YY1 se asocia a un AT más pequeña de las fibras del cuádriceps en pacientes con EPOC, en cuyo músculo también se observa una localización nuclear del factor, a diferencia de los individuos control. La regulación de YY1 parece alterada en la EPOC y podría estar implicada en la atrofia muscular relacionada con la enfermedad.
The atrophy and weakness of the peripheral muscles are negative prognostic factors in chronic obstructive pulmonary disease (COPD).1,2 In order to maintain muscle mass, regeneration of the skeletal muscles is necesary.3 However, based on animal models in COPD, there is evidence that skeletal muscle regeneration may deteriorate as a consequence of systemic inflammation, which contributes to the muscle atrophy.4 We still do not fully understand the molecular mechanisms that may translate into a deterioration of the myogenesis in COPD.
Ying Yang 1 (YY1) is a transcription factor that represses myogenesis that has not previously been researched either in the muscles of COPD patients or, to our knowledge, in the skeletal muscles of healthy adults. Nevertheless, it is known that its expression increases in the lung tissue of COPD patients when compared with control subjects.5 YY1 suppresses the muscle differentiation and gene transcription of the skeletal muscles, such as skeletal alpha actin6 and muscle creatine kinase,7 binding with the pertinent promoters and blocking the binding of a transcription activator, serum response factor.8 For instance, the activation of the nuclear factor kappa B (NF-κB) pathway by tumour necrosis factor-alpha (TNF-α) can inhibit muscle regeneration3 through an increase in YY1 expression.9 Furthermore, the location of YY1 affects its activity, as does its expression. When YY1 is limited to the cytoplasm of the muscle cells, it is inactive, which allows for the differentiation and synthesis of the contractile proteins. YY1 is activated by the transport to the nucleus, for example, as a response to the presence of depolymerised actin.8
There are at least two mechanisms due to which the muscle YYI activity could increase in COPD. First of all, the patients with COPD and muscle wasting may present an increase in the DNA-NF-κB binding in the muscle10 and, therefore, a greater expression of YY1.9 The activation of NF-κB in the quadriceps of COPD patients could be a consequence of the stimulation secondary to the rise in TNF-α in blood11 or in the muscle. However, in patients compared with control subjects, a decrease in TNF-α values in the quadriceps muscle has been reported12 in comparison with the findings in the intercostal muscles.13 Given the fact that TNF-α can also stimulate the activation of satellite cells through the activation of the serum response factor,14 its reduction in the muscles could inhibit muscle regeneration regardless of YY1. In the second place, in the muscle of COPD patients, the activity of YY1 could increase due to the increase in the nuclear transport of YY1 in the presence of a rise in depolymerised actin, a consequence of the accelerated degradation of proteins through the ubiquitin-proteasome pathway.8,15 Therefore, the hypothesis of this present study was that the deregulation of YY1 signalling is involved in the atrophy of the quadriceps of patients with COPD. We have investigated the expression and location of YY1 in the quadriceps muscle of a small group of patients with COPD and control individuals, paired for age, and we have examined the relationship between the expression and location of YY1 and the cross-sectional area (CSA) of the quadriceps fibres.
Individuals and MethodsIndividualsFrom the respiratory department, 15 COPD patients were included for study (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stages II [n=4], III [n=4] and IV [n=7]). The exclusion criteria were heart, kidney or liver failure or a systemic inflammatory, metabolic or neuromuscular disease or a moderate-severe exacerbation (meaning, with the need for antibiotics, oral steroids or hospitalisation) in the previous four weeks. Using an advertisement, 8 healthy control individuals were recruited. All individuals gave their written informed consent and the study was approved by the research committee of the Royal Brompton, Harefield NHS Trust and Ealing and West London Mental Health Trust.
Physiological Determinations and Biopsy of the QuadricepsIn accordance with the guidelines of the American Thoracic/European Respiratory Society, we determined postbronchodilator spirometry,16 lung volumes with plethysmography17 and carbon monoxide diffusing capacity,18 while arterial blood gas was examined from an arterialised blood sample obtained from an earlobe. Lean body mass was determined with bioelectric impedance (Bodystat® 1500, Bodystat, United Kingdom),19 which was corrected for stature to derive the lean body mass index. Physical activity was determined by a triaxial Dynaport® ADL3 accelerometer (McRoberts BV, Netherlands) that the patients used for two days, 12h each day, during normal activity. The mean locomotion time was calculated as previously described.20 The strength of the quadriceps (right leg) was evaluated with the maximum voluntary isometric contraction, in supine decubitus, based on the method by Edwards.21 The percutaneous needle biopsy in the right vastus lateralis was done with local anaesthesia using the Bergstrom technique.22 The samples for the analysis of ribonucleic acid and proteins were immediately frozen in liquid nitrogen, while the histology samples were introduced in pre-cooled isopentane for 15s before being frozen in liquid nitrogen then stored at −80°C.
Analysis of the Muscle BiopsyThere was not enough tissue from each and every individual to complete all the analyses, therefore for each analysis a subgroup of samples was used.
Immunofluorescent Detection of Yin Yang 1The frozen 10μm slices from 10 patients and 8 control individuals were set in a solution of 10% formaldehyde, washed in Triton X-100 at 0.1% in a neutralised saline solution with phosphate buffered saline (PBS) and were blocked with 5% bovine serum albumin (BSA) in PBS, before incubation with rabbit anti-YY1 antibody (dilution 1:400, sc-281; Santa Cruz Biotechnology, United States) and murine anti-heavy chain myosin fast contraction antibody (MYSN02, dilution 1:200, Abcam, United Kingdom) in 3% BSA in PBS for the entire night at 4°C. After an incubation of 1h at room temperature in the dark with secondary antibodies marked with fluorescence (A11008 Invitrogen and A11005 Invitrogen, dilution 1:250 in PBS), the slices were treated with diamino-phenylindole (DAPI). The images (two fields per sample, two cuts per individual) were obtained using a wide-field Zeiss Axiovert microscope, with a 10× lense and Improvision Volocity software. Using a Leica SP2 microscope, we obtained confocal images with a 63× oil immersion lense and they were analysed with LCS Lite software (Leica Microsystems, Germany).
Quantification of the Yin Yang 1 ProteinYY1 is not abundant in the skeletal muscle of adults and, consequently, before immunoblotting, immunoprecipitation was necessary. From nine patients and seven control individuals, for this analysis there were 3–6mg of protein.
Immunoprecipitation of Yin Yang 1Between 3 to 6 mg of homogenised protein were incubated in a solution with Nonidet P40, cocktails of protease inhibitor and phosphatase inhibitor (Sigma, Poole Dorset, United Kingdom) in ice for 30 min with 30μl de Protein G-Sephorose beads. After centrifuging for 2 min (8,000rpm), the supernatant was incubated for 1 h in ice with 1μg of rabbit anti-YY1 antibody (sc-281, Santa Cruz Biotechnologies, United States). For each sample, another 30μl Protein G-Sephorose beads were added and they were re-incubated in ice for 1h. The samples were centrifuged again and the supernatant was discarded. The beads were washed in Nonidet P40 and boiled with 30μl of the sample buffer (loading buffer and 2-mercaptoetanol) for 5 min at 100°C. The process was optimised to guarantee that the supernatant would not have a detectable quantity of YY1 in the Western blot.
Western Blot AnalysisThe samples were analysed using electrophoresis in polyacrylamide gel with sodium dodecyl sulphate (SDS/PAGE) (10% gel) and a semidry immunoblotting technique (LKB). After blocking (5% BSA in PBS), the membranes were incubated with mouse anti-YY1 antibody (ab58066, dilution 1:200, Abcam, United Kingdom) in 3% BSA in PBS for the whole night at 4°C, and immediately followed with an IgG murine antibody bound with horseradish peroxidase (ab 6728, Abcam, United Kingdom, 1:5000 in 3% BSA in PBS) for 1 h at room temperature. The proteins were visualised with Supersignal (Pierce, Rockford, IL, United States), determining the density of the band with a densitometry 1D analysis (AIDA, Raytek, Sheffield, United Kingdom) and was normalised for the quantity of proteins used in the immunoprecipitation.
Determination of the Transversal Area of the Muscle FibresFrom all the subjects, the 10μm frozen transverse muscle slices were incubated with primary antibodies for type I myosin, type IIa myosin and laminin (A4.840 and N2.261 Developmental Studies Hybridoma Bank, University of Iowa, Unites States, and L-9393 Sigma, Zwijndrecht, Netherlands, respectively) followed by secondary antibodies marked with fluorescence (A-21121, A-21426 and A-11069, Invitrogen).23 The epifluorescence signal was registered using a Texas Red excitation filter (540-580nm) for type I myosin, an FITC excitation filter (465-495nm) for type IIa myosin and a UV DAPI excitation filter (340-380nm) for the laminin using a Nikon Eclipse 800 microscope. The fibres were classified into type I, IIa, IIx (without staining) and hybrid I/IIa (dual staining) and we used the laminin edge of the fibres to calculate the CSA of each fibre (and with this the mean CSA for each type of fibre) and the proportions of fibres using Lucia 4.81 statistical software (Nikon, Japan).24 For each individual, we analysed an average of 207 fibres, with a minimum of 103 fibres.
Quantification of the Levels of Tumour Necrosis Factor α mRNAThe TNF-α mRNA transcripts were determined by a chain reaction of the quantitative polymerase, in real time, using SYBR® Green PCR Master Mix (Applied Biosystems) in a 7900HT Fast Real-Time PCR System (Applied Biosystems). The transcripts normalised to a geNorm factor derived from two internal genes, acidic ribosomal phosphoprotein (RPLPO) and beta-2 microglobulin (β2M), as previously reported.25 The following are the primer sequences:
Statistical AnalysisThe data did not have normal distribution (according to the histogram and the test of symmetry), so they are reported as averages (25th percentile and 75th percentile). The group differences in the continuous variables were analysed with the Mann–Whitney U-test, while the Fisher's exact test was used to test the group differences in the categorical variables. Spearman's rank correlation coefficient (ρ) was calculated to determine the relationship among the variables (Statview 1.0, Abacus Instruments). To define the statistical significance, a two-tailed P value ≤.05 was used.
ResultsTable 1, shows the physiological data of the patients and controls. As was expected, the patients presented a deterioration in lung function and a decrease in the strength of the quadriceps, in comparison with the control individuals [maximal voluntary contraction 22 (17.34)kg compared with 34 (28.42)kg in control subjects, P=.03].
Clinical Characteristics of the Patients and Control Subjects.
| COPD Patients (n=15) | Control Subjects (n=8) | |
| Age, years | 69 (63.75) | 69 (65.76) |
| Sex | 53% males | 50% males |
| Tobacco habit | 2 current and 13 ex-smokers | 8 ex-smokers and 2 non-smokers |
| Smoking history, pack/year | 45 (30.75)a | 7 (1.14) |
| FEV1, l | 0.86 (0.59, 1.05)a | 2.92 (2.53, 2.98) |
| FEV1, % reference value | 33 (22, 54)a | 109 (105, 112) |
| TLCO, % reference value | 36 (25, 62)a | 89 (81,99) |
| PaO2, kPa | 9.8 (8.0, 10.4) | 10.4 (8.7, 11.4) |
| Body mass index, kg/m2 | 22.9 (20.7, 26.2) | 24.8 (21.0, 26.7) |
| Lean body mass index, kg/m2 | 16.2 (14.3, 18.6) | 15.8 (14.5, 19.5) |
| MVC of quadriceps, kg | 22 (17, 34)b | 34 (28, 42) |
| Locomotion time, min/12h | 57 (21, 75) | 61 (56, 159) |
The values are means (25th percentile and 75th percentile).
The CSA in the type IIx fibres diminished and the quotient type I fibres: type II fibres decreased in patients with COPD in comparison with control individuals.
In the patients, a significantly smaller CSA was detected in the type IIx fibres than in control individuals [3.439 (2.348, 3.739)μm2 compared with 4.628 (4.087, 6.303)μm2 respectively, P=.05], but there was no significant difference in the CSA of the other types of fibres between groups, as had been previously reported.26 In patients, we detected a significantly lower proportion of type I fibres and a greater proportion of type IIa fibres than in control individuals [34 (14, 39)% compared with 52 (47, 63)% and 60 (49, 70)% compared with 40 (35, 46)%, respectively], as previously described27 (Table 2).
Levels of YY1, HDAC 5, and HDAC 5 Bound With YY1 and Mean CSA of the Type I, I/IIa, IIa and IIx Fibres in the Quadriceps of Patients With COPD and Control Individuals.
| COPD Patients (n=15) | Control Subjects (n=8) | |
| YY1, AU/mg protein | 3.25 (1.95, 7.17) (n=9) | 3.84 (2.18, 6.01) (n=7) |
| Levels of mRNA-TNF-α, AU | 0.08 (0.03, 0.11) (n=15) | 0.04 (0.03, 0.05) (n=8) |
| CSA type I fibre, μm2 | 5352 (4151, 6783) (n=15) | 5.644 (4.111, 7.411) (n=8) |
| CSA type I/IIa fibre, μm2 | 4971 (3292, 7309) (n=15) | 5754 (5005, 5956) (n=3) |
| CSA type IIa fibre, μm2 | 4221 (2996, 5170) (n=15) | 3314 (3002, 5408) (n=15) |
| CSA type IIx fibre, μm2 | 3439 (2348, 3739)b (n=14) | 5015 (3618, 6303) (n=8) |
| Percentage of type I fibres | 34 (11, 38)a (n=15) | 52 (47, 63) (n=8) |
| Percentage of type I/IIa fibres | 3 (1, 6)a (n=15) | 0 (0, 4) (n=8) |
| Percentage of type IIa fibres | 60 (52, 70)a (n=15) | 40 (35, 46) (n=8) |
| Percentage of type IIx fibres | 3 (1, 16)a (n=15) | 1 (0, 4) (n=8) |
The values are means (25th percentile, 75th percentile). The percentages of fibres in each individual reach 100% (the sum of the means in each group does not necessarily equal 100% due to the variance around the mean). The levels for MRNA normalise for the transcripts of RPLPO (internal gene).
The levels of YY1 in the quadriceps were associated with a decrease of the CSA in the muscle fibres in COPD.
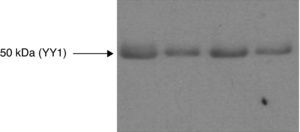
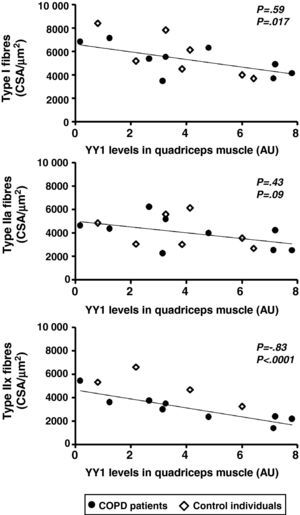
The quantity of YY1 protein in the quadriceps was not significantly different in patients compared with the control subjects (Fig. 1, Table 2). In patients and controls, the levels of YY1 protein inversely correlated with the CSA of the type IIx fibres (ρ=−0.83, P<.0001); likewise it was true with the CSA of the type I fibres (ρ=−0.59, P=.017), and there was also an observed tendency towards a negative correlation with the CSA of the type IIa fibres (ρ=−0.43, P=.09) (Fig. 2). Only in the group of patients did we identify a correlation between the levels of YY1 and in the muscle the CSA of the type IIx fibres (ρ=−0.88, P=.002). The muscle levels of YY1 did not correlate with the forced expiratory volume in one second or the CO2 diffusing capacity (TLCO) as a percentage of the reference value, maximal voluntary contraction of the quadriceps or the lean mass index in patients and control subjects (P=.69, .43, .94 and .21, respectively) or correlation was only identified in the patient group.
Western blot analysis for YY1 from the quadriceps muscle of COPD patients and healthy control subjects. Immunoblotting representative of YY1 in muscle samples of a control subject (left) and three COPD patients (right), subjected to immunoprecipitation using an anti-YY1 antibody. The bands are observed in position 50kDa. Statistically significant differences in the YY1 protein levels were not identified in the muscle of the patients compared with the control individuals.
Point diagrams of the YY1 protein levels and CSA of the type I, IIa y IIx fibres in the quadriceps of patients with COPD and healthy control subjects.
There were negative correlations between the YY1 protein levels and the CSA of the type I and IIx fibres and a tendency towards a negative correlation between the levels of the factor and the CSA of the type IIa fibres when the patients and the control subjects were combined (ρ=−0.83, P<.0001; ρ=−0.59, P=.017 and ρ=−0.43, P=.09, respectively), and a negative correlation between the levels of YY1 and the CSA of the type IIx fibres was only detected in patients (ρ=−0.88, P=0.002).
The muscle TNF-α mRNA levels were higher in patients than in control subjects (0.08 [0.03, 0.1] arbitrary units [AU] compared with 0.04 [0.03, 0.05] AU, P=.05). The TNF-α mRNA transcripts and the levels of YY1 did not correlate in patients and control subjects (P=.38) or in the patients alone.
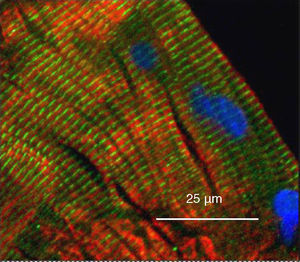
Nuclear Localisation of Yin Yang 1 in the Muscle of a Patient Subgroup With Chronic Obstructive Pulmonary Disease but not in Control IndividualsYY1 was absent from the nucleus but present in the cytoplasm in the quadriceps of the healthy control subjects (Figs. 3 and 4C, D, G, H). YY1 in the cytoplasm aligned with the sarcomeres, but it did not co-localise with myosin (Fig. 3).
Image from a wide-field microscope of a longitudinal slice of the quadriceps muscle of a healthy control subject demonstrating the localisation of YY1 regarding the muscle nuclei by means of immunohistochemistry. The muscle was stained for YY1 (green), the heavy chain of the fast myosin (red) and the nuclei using 4′,6-diamidino-2-phenylindole (blue). The YY1 staining is observed along the sarcomeres, not co-localised with the myosin, and the absence of nuclei is also observed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
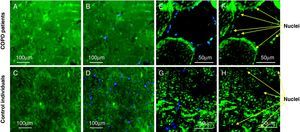
Microscope images of quadriceps muscle slices from COPD patients and control subjects, demonstrating in the myotubules the localisation of YY1 with immunohistochemistry with regard to the muscle nuclei. (A–D) Wide-field microscope images. E-H: images from a confocal microscope with cross-sectional quadriceps muscle cuts that demonstrate the YY1 stain in green and for the nuclei with 4′,6-diamidino-2-phenylindole in blue (B, D, E, and G) and the same cuts that show staining for YY1 only in green (A, C, F, and H). (A, B, E, and F) The slices of a patient in whom the anomalous staining pattern of the factor was identified in the nucleus as well as in the cytoplasm. (C, D, G, and H) From a control subject showing exclusively cytoplasmic YY1 staining.
YY1 staining was demonstrated in the nuclear region in none of the control individuals but in 5 of the 10 patients. This difference between groups was statistically significant (Fisher's exact test, P=.04). Compared with 6 of the 8 control subjects, only in 2 of the 10 patients was there a demonstrated exclusively cytoplasmic YY1 distribution that, once again, was statistically significant (Fisher's exact test, P=.05). No significant difference was identified between patients with or without nuclear YY1 localisation regarding muscle or lung function, quadriceps fibre CSA or YY1 protein levels.
DiscussionWe have made a new finding: that the YY1 transcription factor, a repressor of muscle-specific gene expression and myogenesis, is expressed in a proportion inverse to CSA of the type IIx and type I fibres in the quadriceps muscle of patients with COPD. In a significant proportion of these patients, the nuclear localisation of the factor was evident, which contrasts with the distribution in the muscle of healthy adults.
Critique of the methodNaturally, the present study presents limitations. First of all, the size of the sample was small and the associations detected had to stand out; therefore it is possible that no other associations or group differences were identified due to the lack of power. The control subjects included in the present study were characterised by a relatively low lean mass index and a wide variance in their degree of physical activity, for which there is no clear explanation, which increases the threshold for the statistical significance of the group differences. Although the study of patients in stage II, III, and IV of the GOLD initiative should have made it easier to find correlations between the muscle and lung function and the YY1 levels (because the data was not expected to gather), the exclusive use of patients in stage IV could have maximised the probability of finding a significant difference in YY1 expression between patients and control subjects. Second, the observational design does not allow for the extraction of conclusions about the effect of the differences in YY1 on muscle structure and function, which merely highlights an association between the increase in expression of the factor and the smaller size of the fibres and between the nuclear localisation of the factor and the presence of COPD and less strength in the quadriceps muscle than in control individuals. Lastly, we used the localisation and expression of the factor as an indirect variable of the activity because YY1 is activated by transport from the cytoplasm to the nucleus. Our findings could have been corroborated by the quantification of YY1 bound to DNA (immunoprecipitation of chromatin, a technique that has not been previously published as being used in human muscle) or the quantification of YY1 able to bind with DNA (for example, by means of electrophoretic analysis of the change in mobility).
It could also be argued that the YY1 factor detected in the nuclei is not present in the periphery of the myofibrils, but is instead in the latent, satellite cells. However, we consider that this is improbable as the satellite cells only represent 2%-5% of the muscle nuclei.28 Even supposing a massive increase in the population of satellite cells in the tissue of the patients, the number of nuclei that stained for the factor considerably surpasses the number that could be justified by the satellite cells. Furthermore, in the samples of this present study, the absence of centralised nuclei suggests a limited activation of the satellite cells in the period of time when the samples were taken.
Significance of the FindingsThe implication of a powerful inverse correlation between the levels of the YY1 factor and the size of the fibres is that YY1 could participate in the mechanism of fibre atrophy, particularly as the relationship is stronger with the CSA of the type IIx fibres and these fibres seem to be the type that atrophy the most in COPD.26 However, this hypothesis requires additional research, for example, examining the effect in the skeletal muscle of adult knockout or knockdown mice with emphysema induced by tobacco smoke or other means.
The finding that patients with a nuclear localisation of the factor do not necessarily have higher levels can be explained by the fact that its localisation and expression can be regulated independently. It is possible that the patients with a greater expression of YY1 and a smaller size of the fibres are characterised by greater levels of NF-κB activation in the muscle, while in others a greater reserve of depolymerised actin would give rise to a nuclear accumulation of the factor without an increase in its expression. However, we did not find a correlation between the local values of TNF-α and YY1 in the muscle of the patients, which supports the suggestion that the levels of the factor are mainly determined by local TNF-α that would activate the NF-κB factor.
In conclusion, we report the new finding that YY1, transcription factor for the repression of the specific gene expression of muscles and myogenesis, is expressed associated with a decrease in the CSA of type IIx and type I fibres of the quadriceps muscle in COPD. YY1 is also localised in the nuclei of the quadriceps of COPD patients in comparison with healthy control subjects, paired by age. We speculate that the greater activity of YY1 could be implicated in the deterioration of skeletal muscle regeneration and the atrophy of the muscle fibres in COPD.
Conflict of InterestS.A. Natanek (born in Sathyapala) receives funding from a Wellcome Trust Clinical Research Training Fellowship and previously received a grant from GlaxoSmithKline. G.S. Marsh and J. Riddoch-Contreras received funding through a scholarship at the Imperial College by GlaxoSmithKline. W.D.-C. Man receives funding from the National Institute for Health Research Clinician Scientist Award. GlaxoSmithKline took no part in either the compiling of the data or preparation of the manuscript. The NIHR Respiratory Biomedical Research Unit of the Royal Brompton Hospital and Imperial College contribute to a part of M.I. Polkey's salary.
We would like to thank Derek Cramer and the Lung Function Department of the Royal Brompton Hospital for the completion of the lung function tests; Professor David Hansell and the CT Department of the Royal Brompton Hospital for performing the thick cuts; Kathleen Daenen at the University Hospital of Maastricht for the examination of the frozen slices; Gert Schaart and Lex Verdijk from Maastricht University for their help with immunofluorescence for the detection of the type and size of the fibres; and Harry Gosker and Ramon Langen from Maastricht University for their comments on the manuscript.
Please cite this article as: Natanek SA, et al. Expresión y localización del factor de transcripción Yin Yang 1 en el músculo cuádriceps en la enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2011;47:296–302.