In recent years, several studies have highlighted the relationship between physical activity (PA) in chronic obstructive pulmonary disease (COPD) and a lower risk of exacerbations, hospitalization, and death.1,2 While associations have been established between respiratory function and muscle strength, and between muscle strength and the level of PA,3,4 the influence of PA has been consistently observed in different individual characteristics, geographic settings, and instruments for measuring physical activity, irrespective of spirometric severity and other predictors of COPD progress.5 Interest is now growing in encouraging COPD patients to exercise, although the evidence available to date is still limited.6 Several questions need to be answered, and simple, inexpensive strategies that could be implemented in the community to promote PA in these patients need to be identified, since improved functional capacity and quality of life achieved by respiratory rehabilitation programs rarely translate into increased PA in this population.7 In this respect, in 2009 our group drew up the initial Walking Guide for COPD Patients. This guide, available in print and online (www.pasearconepoc.es), has been updated several times since then, and now includes 94 routes in 40 walking areas in and around our city. The walks are divided into five levels of difficulty, in terms of distance and incline, and disease severity and other comorbidities were taken into account to draw up an arbitrary table to help select suitable walks (see website).
The aim of this study was to evaluate the possible clinical efficacy of a walking program in COPD patients. This was a prospective, observational non-randomized, open-label, unblinded study, comparing an intervention group with a reference group. Patients in the intervention group followed the indications of our walking guide if they lived in or around the city, and if not, they were prescribed a similar program to that of the guide. All patients in this group kept a daily record of symptoms and exacerbations and were contacted monthly by telephone to ask about their mean monthly compliance with the program. The control group, who were also recruited in respiratory medicine clinics and previously screened, followed the standard recommendations for PA given in the clinic. None of the patients included in this group had refused to participate in the walking program. Correct treatment for their disease and therapeutic compliance were confirmed in both study groups. In both groups, the variables listed in the table were determined at baseline and after 1 and 2 years of follow-up, except for moderate and/or severe exacerbations, which were quantified globally in a 2-year period prior to the study start, and during the 2-year study period. Normal distribution was confirmed, and difference in means was analyzed using the Student's t-test and, given the sample size, results were confirmed with the corresponding non-parametric tests.
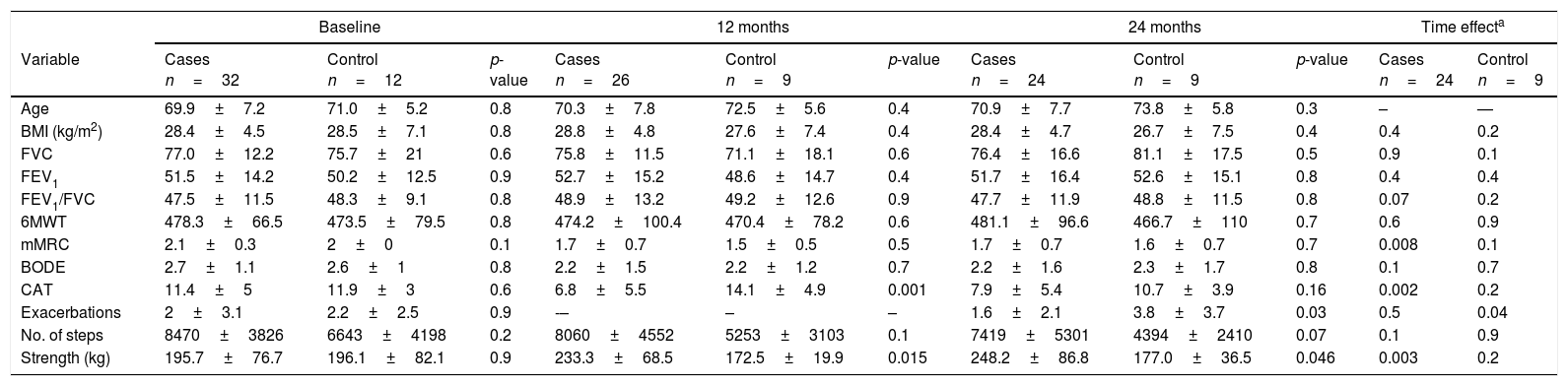
A total of 44 patients were recruited from the respiratory medicine clinic (32 in the intervention group and 12 in the control group). Eleven participants did not complete the protocol: eight in the intervention group (five drop-outs, one deterioration, and two deaths); and three in the control group (three drop-outs). The table shows results at baseline, at 1 year and at 2 years, and the time effect in each group between baseline and 2 years. Before analysis, the population that completed the study was determined to be similar to the baseline population. The only changes detected between the two groups in the first year of follow-up were a significantly greater reduction in the CAT score in the intervention group, which was not maintained at 2 years, and an improvement in lower limb strength, which was maintained. Differences were also observed in the number of exacerbations, in favor of the group that followed the walking program. As for the time effect, the intervention group showed a significant reduction in dyspnea and CAT score and improved lower limb strength; there was a smaller reduction in number of steps at 2 years in the intervention group that did not reach statistical significance (p = 0.07); significantly more exacerbations occurred in the control group.
The quality of evidence on interventions aimed at increasing PA in patients with COPD is still low.5 Despite being a small, non-randomized series, our study revealed improvements in quality of life and a reduced frequency of exacerbation that, while modest, would support the usefulness of simple walking programs in COPD. In order to help physicians prescribe the program and to make it more accessible to patients, our walking guide was initially published in print and online. Recently, Moy et al.8 showed that PA levels can be increased with the use of motivational techniques and positive reinforcement, but only in the short term. In addition to the walking guide itself, our program also consisted of a daily record of symptoms and motivating phone calls. Translation of this approach to routine clinical practice may be difficult in many settings, raising questions regarding feasibility. Our experience prompted us to design a cellphone application (“Paseos COPD”) which completes the original guide with motivational and record-keeping tools, and information for physicians. Assessment is pending, but we hope that it will improve results. Given the demonstrated evidence of the importance of physical activity in COPD, we believe that initiatives of this type should be implemented in routine clinical practice (Table 1).
Results at 1 year and 2 years and time effect.
| Baseline | 12 months | 24 months | Time effecta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Cases n=32 | Control n=12 | p-value | Cases n=26 | Control n=9 | p-value | Cases n=24 | Control n=9 | p-value | Cases n=24 | Control n=9 |
| Age | 69.9±7.2 | 71.0±5.2 | 0.8 | 70.3±7.8 | 72.5±5.6 | 0.4 | 70.9±7.7 | 73.8±5.8 | 0.3 | – | — |
| BMI (kg/m2) | 28.4±4.5 | 28.5±7.1 | 0.8 | 28.8±4.8 | 27.6±7.4 | 0.4 | 28.4±4.7 | 26.7±7.5 | 0.4 | 0.4 | 0.2 |
| FVC | 77.0±12.2 | 75.7±21 | 0.6 | 75.8±11.5 | 71.1±18.1 | 0.6 | 76.4±16.6 | 81.1±17.5 | 0.5 | 0.9 | 0.1 |
| FEV1 | 51.5±14.2 | 50.2±12.5 | 0.9 | 52.7±15.2 | 48.6±14.7 | 0.4 | 51.7±16.4 | 52.6±15.1 | 0.8 | 0.4 | 0.4 |
| FEV1/FVC | 47.5±11.5 | 48.3±9.1 | 0.8 | 48.9±13.2 | 49.2±12.6 | 0.9 | 47.7±11.9 | 48.8±11.5 | 0.8 | 0.07 | 0.2 |
| 6MWT | 478.3±66.5 | 473.5±79.5 | 0.8 | 474.2±100.4 | 470.4±78.2 | 0.6 | 481.1±96.6 | 466.7±110 | 0.7 | 0.6 | 0.9 |
| mMRC | 2.1±0.3 | 2±0 | 0.1 | 1.7±0.7 | 1.5±0.5 | 0.5 | 1.7±0.7 | 1.6±0.7 | 0.7 | 0.008 | 0.1 |
| BODE | 2.7±1.1 | 2.6±1 | 0.8 | 2.2±1.5 | 2.2±1.2 | 0.7 | 2.2±1.6 | 2.3±1.7 | 0.8 | 0.1 | 0.7 |
| CAT | 11.4±5 | 11.9±3 | 0.6 | 6.8±5.5 | 14.1±4.9 | 0.001 | 7.9±5.4 | 10.7±3.9 | 0.16 | 0.002 | 0.2 |
| Exacerbations | 2±3.1 | 2.2±2.5 | 0.9 | -– | – | – | 1.6±2.1 | 3.8±3.7 | 0.03 | 0.5 | 0.04 |
| No. of steps | 8470±3826 | 6643±4198 | 0.2 | 8060±4552 | 5253±3103 | 0.1 | 7419±5301 | 4394±2410 | 0.07 | 0.1 | 0.9 |
| Strength (kg) | 195.7±76.7 | 196.1±82.1 | 0.9 | 233.3±68.5 | 172.5±19.9 | 0.015 | 248.2±86.8 | 177.0±36.5 | 0.046 | 0.003 | 0.2 |
6MWT: 6-minute walk test; BMI, body mass index; BODE: Body mass index, airflow obstruction, Dyspnea and Exercise capacity; CAT: COPD Assessment Test; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; mMRC: modified British Medical Council dyspnea scale; No. of steps: daily steps quantified with accelerometer (ActiGraph, the accelerometer was analyzed with ≥4 days of ≥10h/day recording); Strength: maximum lower limb strength (maximum 1 repetition) in leg press exercise.
This study was funded by a research project from the Ministry of Economy and Competitiveness of the Government of Spain (DEP2011-30042).
Please cite this article as: Cebollero P, Antón M, Hernández M, Hueto J. Programa de paseos para pacientes con EPOC: impacto clínico tras 2 años de seguimiento. Arch Bronconeumol. 2018;54:439–440.