Measuring predicted post-operative diffusion capacity of the lung for carbon monoxide (ppoDLCO) is essential to determine patient operability and to stratify the risk of patients who are candidates for major lung cancer surgery. Studies that established surgical risk variables were based on open surgery series. The aim of our study was to analyze morbidity and mortality as a function of ppoDLCO and to compare its behavior in open and video-assisted thoracic surgery (VATS).
MethodsWe compared 90-day mortality and morbidity in patients undergoing open surgery versus VATS as a function of decline in ppoDLCO. Propensity score matching (using age, ASA, arterial vascular disease, BMI, gender, stage, ppoDLCO, and ppoFEV1) was applied to create comparable open surgery and VATS groups.
ResultsOf 2,530 patients with lung cancer and ppoDLCO values, a sample of 1,624 (812 per group) was obtained after score matching. The relative risk of mortality associated with thoracotomy in patients with ppoDLCO < 60 is 2.66 (p < 0.02) compared to VATS. The risk of thoracotomy in terms of overall and cardiac and respiratory morbidity is higher than that of VATS for almost all ppoDLCO values.
ConclusionsMajor resection by VATS shows lower morbidity and mortality in patients with the same ppoDLCO. A steady rise in the risk of mortality begins to occur at higher ppoDLCO values in thoracotomy (∼60) than in VATS (∼45).
La medición de la capacidad de difusión del carbono monóxido postoperatoro (ppoDLCO) es esencial para la operabilidad del paciente y la estratificación del riesgo de los pacientes subsidiarios de una resección pulmonar mayor por cáncer. Los estudios que fijan los límites de riesgo quirúrgico se basan en series de cirugía abierta. El objetivo de nuestro trabajo es analizar la morbilidad y mortalidad en relación a la ppoDLCO y comparar su comportamiento en cirugía abierta y cirugía torácica videoasistida (VATS).
MétodosComparación de la mortalidad a 90 días y la morbilidad en pacientes intervenidos por cirugía abierta frente a videoasistida en relación al descenso de la ppoDLCO. Emparejamiento por puntaje de propensión (variables: edad, ASA, vasculopatía arterial, IMC, sexo, estadio, ppoDLCO y ppoFEV1) para realizar grupos comparables entre abierta y VATS.
ResultadosDe 2.530 pacientes con cáncer de pulmón y medición de ppoDLCO, se obtiene tras el pareamiento por puntaje una muestra de 1.624 (812 por grupo). El riesgo relativo de mortalidad de la toracotomía para una ppoDLCO <60 es de 2,66 (p < 0,02) respecto a la videocirugía. Tanto para morbilidad total como para la cardíaca y respiratoria, el riesgo de la toracotomía es superior a la videocirugía para casi todos los valores de ppoDLCO.
ConclusionesLa resección mayor por VATS muestra una morbimortalidad inferior para una misma ppoDLCO. El aumento continuo del riesgo de mortalidad empieza a darse en valores de ppoDLCO superiores en toracotomía (∼60) que en VATS (∼45).
Respiratory function tests are still a basic component of preoperative studies in pulmonary resection. These tests include diffusing capacity of the lung for carbon monoxide (DLCO) and predicted post-operative DLCO (ppoDLCO), determinations that are considered essential to stratify risk in patients with and without COPD1,2. Moreover, these methods are recommended for risk stratification in preoperative study guidelines3,4. However, most of the studies on preoperative tests are conducted in patients undergoing thoracotomy, while few have been published on patients undergoing minimally invasive surgery4–6. In predictive models such as EUROLUNG, which include video-assisted thoracic surgeries (VATS), this approach has resulted in a lower rate of complications than open surgery7, and some studies show that even patients with low DLCO can safely undergo minimally invasive surgery8.
Using the national database of the Spanish Video-assisted Thoracic Surgery Group (GEVATS)9, we analyzed whether a thoracoscopic approach is associated with a different morbidity and mortality curve for ppDLCO than open surgery.
Materials and methodsWe searched the GEVATS database to find data from patients undergoing anatomical pulmonary resection between December 12, 2016 and March 20, 20188. GERVATS is a resource sponsored by the Spanish Society of Thoracic Surgery that prospectively registers patients from 33 thoracic surgery centers.The study has been approved by the ethics committees of all participating centers.
For the automatic calculation of ppoDLCO, the number of segments resected in previous surgeries was taken into account, using the anatomical method: ppoDLCO = DLCO × (19 – resected segments in previous surgeries – resected functioning segments/19 – non-functioning segments – resected segments in previous surgeries).
In-hospital mortality and 90-day mortality were defined as outcome variables. Atrial arrhythmia, heart failure, acute myocardial infarction, stroke, and deep vein thrombosis were defined as cardiac complications. Respiratory complications were defined as prolonged mechanical ventilation, re-intubation, prolonged air leak > 5 days, atelectasis, pneumothorax and/or pleural effusion, pneumonia, adult respiratory distress syndrome, bronchopleural fistula, empyema, chylothorax and pulmonary thromboembolism. Two variables were created, one that included all respiratory complications and another that excluded air leaks, bronchopleural fistula, and chylothorax. This subdivision was made with the aim of reducing the weight of air leak, bronchopleural fistula, and chylothorax from other more serious respiratory complications, since these events are associated more with the surgical technique than with the patient’s ppoDLCO.
Continuous preoperative variables were described using mean and standard deviation, and categorical variables used frequency and percentage. Continuous preoperative variables were compared using the Wilcoxon signed-rank test, since in no case did the 2 categories compared show normality. Categorical variables were compared using Fisher’s exact test. The cut-off point for statistical significance was p < 0.05.
Propensity score matching was performed for each morbidity and mortality variable to compare the differences between open surgery and VATS, using the Optimal matching method in the MatchIt library in R10, which finds the matched samples with the smallest average absolute distance across all matching pairs. The distances between the samples of the 2 groups were calculated using the logit method. Unpaired and paired patients with a distance greater than 0.05 were excluded. The variables used for propensity score matching were selected according to their clinical importance and statistical significance: age, American Society of Anesthesiologist (ASA) classification, arterial vascular disease, body mass index (BMI), sex, predicted post-operative forced expiratory volume in 1 second (ppoFEV1), ppoDLCO, and TNM stage.
To calculate the proportion of morbidity and mortality variable events according to ppoDLCO, centered moving average smoothing was performed with a range of ± 5 ppoDLCO values.
ResultsOf the 3,533 cases reported in the database, 3,085 had a diagnosis of lung cancer, and DLCO data were available in 2,530 (82%) of these cases. The latter group were the series analyzed in this study. First, the baseline variables of the patients and their distribution between open surgery and VATS surgery were described.
Study patient characteristics are shown in Table 1.
Baseline patient characteristics.
| Variable | Total (n = 2,530) | Open (n = 1,060) | VATS (n = 1,470) | p |
|---|---|---|---|---|
| Age, mean (SD) | 65.07 (9.9) | 64.71 (10.3) | 65.33 (9.6) | 0.306 |
| Sex, n (%) | <0.001 | |||
| Women | 767 (30.3) | 272 (25.7) | 495 (33.7) | |
| Men | 1,763 (69.7) | 788 (74.3) | 975 (66.3) | |
| BMI, mean (SD) | 26.957 (4.6) | 26.965 (4.5) | 26.952 (4.6) | 0.825 |
| Smoking, n (%) | 0.208 | |||
| Smoker | 709 (28.5) | 295 (28.4) | 414 (28.5) | |
| Former smoker (<1 year) | 326 (13.1) | 136 (13.1) | 190 (13.1) | |
| Former smoker (>1 year) | 1,078 (43.3) | 468 (45.0) | 610 (42.0) | |
| Never-smoker | 377 (15.1) | 140 (13.5) | 237 (16.3) | |
| AHT, n (%) | 0.180 | |||
| Yes | 1,111 (43.9) | 449 (42.4) | 662 (45.1) | |
| No | 1,417 (56.1) | 611 (57.6) | 806 (54.1) | |
| Heart failure, n (%) | 0.666 | |||
| Yes | 60 (2.4) | 26 (2.4) | 34 (2.3) | |
| No | 2,469 (97.6) | 1,033 (97.5) | 1,436 (97.7) | |
| Ischemic heart disease, n (%) | 0.666 | |||
| Yes | 218 (8.6) | 88 (8.3) | 130 (8.8) | |
| No | 2,312 (91.4) | 972 (91.7) | 1,340 (91.2) | |
| Arrhythmia, n (%) | 0.296 | |||
| Yes | 200 (7.9) | 91 (8.6) | 109 (7.4) | |
| No | 2,329 (92.1) | 968 (91.4) | 1,361 (92.6) | |
| TIA, n (%) | 0.776 | |||
| Yes | 120 (4.7) | 52 (4.9) | 68 (4.6) | |
| No | 2,409 (95.3) | 1,007 (95.1) | 1,402 (95.4) | |
| Arterial vascular disease, n (%) | 0.888 | |||
| Yes | 225 (8.9) | 93 (8.8) | 132 (9.0) | |
| No | 2,304 (91.1) | 966 (91.2) | 1,338 (91.0) | |
| Diabetes, n (%) | 0.084 | |||
| Type 1 | 44 (1.7) | 25 (2.4) | 19 (1.3) | |
| Type 2 | 414 (16.4) | 181 (17.1) | 233 (15.8) | |
| No | 2,071 (81.9) | 853 (80.5) | 1,218 (82.9) | |
| Creatinine levels > 2, n (%) | 0.334 | |||
| Yes | 72 (2.8) | 26 (2.5) | 46 (3.1) | |
| No | 2,458 (97.2) | 1,034 (97.5) | 1,424 (96.9) | |
| FEV1 (%), mean (SD) | 89.431 (20.2) | 85.852 (18.9) | 92.016 (20.7) | <0.001 |
| FVC (%), mean (SD) | 97.135 (19.1) | 94.321 (18.1) | 99.174 (19.5) | <0.001 |
| DLCO (%), mean (SD) | 83.356 (20.9) | 81.912 (20.7) | 84.397 (21.0) | <0.001 |
| TNM stage, n (%) | <0.001 | |||
| 0-II | 1,803 (82.4) | 691 (75.0) | 1,112 (87.7) | |
| III- IV | 386 (17.6) | 230 (25.0) | 156 (12.3) | |
| ASA, n (%) | 0.021 | |||
| I | 54 (2.1) | 19 (1.8) | 35 (2.4) | |
| II | 1,069 (42.4) | 414 (39.2) | 655 (44.6) | |
| III | 1,338 (53.0) | 593 (56.2) | 745 (50.8) | |
| IV | 62 (2.5) | 30 (2.8) | 32 (2.2) | |
| Surgery time (minutes), mean (DE) | 183.17 (69.4) | 196.99 (76.8) | 173.25 (61.8) | <0.001 |
AHT: arterial hypertension; ASA: American Society of Anesthesiologists classification; BMI: body mass index; DLCO: lung diffusion capacity; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; SD: standard deviation; TIA: transient ischemic attack; VATS: video-assisted thoracic surgery.
Bold values signifies which are statistically significant.
These data show that both mean age and BMI are similar in both groups. The most frequently observed comorbidities are arterial hypertension (AHT) (44.5%) and diabetes mellitus (DM) (18.5%); these were similarly distributed in both groups. Other comorbidities were observed in less than 10% of patients and showed a similar distribution.
However, differences in staging were observed: 68% of the VATS group were stage I, compared to 44% in the thoracotomy group. Similarly, the percentage of stage IIIs was 23.5% in the thoracotomy group, but just under 11% in the VATS group.
Remarkably, cardiac complications are seen in 6%, most of which were atrial arrhythmias (70.6% of all cardiac complications; 4.5% of the patients in the study series). Significant differences were observed in both atrial arrhythmia and heart failure, with a higher rate in the open surgery group.
Respiratory complications were reported in 23%, mostly air leaks (13%), pneumonia (4.5%), and atelectasis (3.6%). Significantly more respiratory complications (excluding air leak, pneumothorax and/or effusion, and chylothorax) were observed in the thoracotomy group.
To create more comparable groups, patients in the thoracotomy and VATS groups were matched by propensity score. We obtained 2 comparable groups for the variables considered clinically relevant (age, arterial vascular disease, and BMI) and for the variables that were allocated asymmetrically with significant differences between the thoracotomies and VATS groups in our sample (ASA, sex, TNM stage). After propensity score matching, a sample of 812 patients per group was obtained (total n = 1,624). Table 2 shows the characteristics of the variables of the sample obtained after score matching and compares the standardized differences and negative p-values of the new sample with the values obtained from the sample before pairing.
Characteristics of variables after propensity score matching.
| Variable | Sample after score matching | Sample before score matching | ||||
|---|---|---|---|---|---|---|
| Open (n = 812) | VATS (n = 812) | p | Standardized difference | p- | Standardized difference | |
| Age, mean (SD) | 65.43 (10.3) | 65.60 (9.0) | 0.589 | −0.016 | 0.307 | −0.072 |
| ASA, n (%) | 0.021 | |||||
| I | 14 (1.7) | 13 (1.6) | 0.010 | −0.045 | ||
| II | 332 (40.9) | 318 (39.2) | 0.885 | 0.035 | −0.100 | |
| III | 445 (54.8) | 461 (56.8) | −0.040 | 0.098 | ||
| IV | 21 (2.6) | 20 (2.5) | 0.008 | 0.034 | ||
| Arterial vascular disease, n (%) | −0.017 | 0.888 | −0.013 | |||
| Yes | 79 (9.7) | 83 (10.2) | 0.804 | |||
| No | 733 (90.3) | 729 (89.8) | ||||
| BMI, mean (SD) | 26.89 (4.5) | 26.83 (4.6) | 0.616 | 0.014 | 0.825 | −0.019 |
| Sex, n (%) | 0.006 | <0.001 | 0.185 | |||
| Women | 208 (25.6) | 210 (25.9) | 0.955 | |||
| Men | 604 (74.4) | 602 (74.1) | ||||
| TNM stage, n (%) | 0.082 | 0.086 | <0.001 | 0.295 | ||
| 0-II | 651 (80.2) | 679 (83.6) | ||||
| III- IV | 161 (19.8) | 133 (16.4) | ||||
| ppoFEV1, mean (SD) | 69.75 (16.9) | 69.70 (16.0) | 0.954 | 0.004 | <0.001 | −0.288 |
| ppoDLCO, mean (SD) | 66.04 (17.6) | 66.18 (18.0) | 0.952 | −0.008 | <0.001 | −0.148 |
ASA: American Society of Anesthesiologists; BMI: body mass index; ppoDLCO: post-operative predicted carbon monoxide diffusion capacity; ppoFEV1: post-operative predicted forced expiratory volume in 1 s; SD: standard deviation; VATS: video-assisted thoracic surgery.
The standardized difference is calculated by dividing the difference in the averages of the 2 groups by the standard deviation of the total population. For these purposes, categorical variables (ASA) and binary variables (sex, stage TNM) are treated as dummy variables.
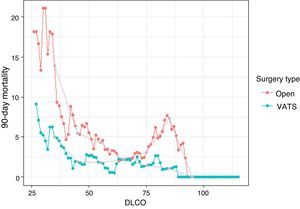
A comparative study of 90-day mortality between VATS and thoracotomy according to ppoDLCO levels was conducted (Fig. 1). Both curves show an increase in the 90-day mortality rate as the ppoDLCO decreases to reach risk values. The mortality curve of thoracotomy patients begins to rise when DLCO values reach around 60, and around 45 in VATS-operated patients. The difference becomes greater as ppoDLCO values of 30 are reached. The relative risk (RR) is 2,778 (p = 0.24) and 2.83 (p = 0.26) in the ppoDLCO ranges [30–40] and [40–50], although differences are not significant. The RR is lower for the remaining ranges, except for ppoDLCO values of around 80 in the thoracotomy group, which show an increase in the proportion of 90-day mortality events.
Proportions of patients who died after 90 days of the intervention obtained after calculating the centered moving average with a range of ± 5 values. Only the proportions of values that were calculated from at least 10 patients are shown. Gray dots show the results of the moving average at values 35, 45, 55, 65, 75, 85 and 95. The results shown by the gray dots are obtained without overlapping the population at different points.
Table 3 shows the comparison between 90-day mortality at various ppoDLCO cut-off points for both techniques.
Mortality at 90 days at different ppoDLCO cut-off points.
| ppoDLCO | Proportion of events (%) | p | RR | Sample size | ||
|---|---|---|---|---|---|---|
| Open | VATS | Open | VATS | |||
| <30 | 1 (12.5) | 0 (0) | 1 | Inf. | 8 | 5 |
| <35 | 4 (21.1) | 1 (5.3) | 0.33977 | 4 | 19 | 19 |
| <40 | 6 (13.6) | 2 (4.4) | 0.1572 | 3.068 | 44 | 45 |
| <45 | 7 (8.4) | 3 (3.4) | 0.2037 | 2.446 | 83 | 87 |
| <50 | 11 (8.1) | 4 (2.7) | 0,06063* | 3.015 | 135 | 148 |
| <55 | 14 (6.7) | 7 (2.9) | 0,07381* | 2.278 | 209 | 238 |
| <60 | 19 (5.9) | 7 (2.2) | 0.02602** | 2.655 | 320 | 313 |
| <65 | 21 (5) | 8 (1.9) | 0.02182** | 2.594 | 420 | 415 |
| <70 | 23 (4.6) | 11 (2.2) | 0.03713** | 2.104 | 497 | 500 |
| <75 | 26 (4.4) | 11 (1.9) | 0.0183** | 2.327 | 588 | 579 |
| <80 | 28 (4.3) | 13 (2.0) | 0.02587** | 2.088 | 658 | 638 |
| <85 | 32 (4.5) | 14 (2.0) | 0.01039** | 2.225 | 710 | 691 |
| <90 | 34 (4.6) | 14 (1.9) | 0.00485** | 2.392 | 740 | 729 |
| <95 | 34 (4.5) | 14 (1.9) | 0.00482** | 2.413 | 760 | 755 |
ppoDLCO: predicted post-operative carbon monoxide diffusion capacity; RR: relative risk; VATS: video-assisted thoracic surgery.
The table shows the results of comparing 90-day mortality distributions among the 2 groups for patients with ppoDLCO values less than different cut-off points. Comparison metrics are Fisher's exact test and relative risk. Rows with asterisks show differences between groups with a tendency towards significance.
Significant differences are observed in the risk of 90-day mortality between patients undergoing VATS or thoracotomy. Groups of patients with ppoDLCO below 30, 35, 40, 45, 50, and 55 show no differences between thoracotomy and VATS due to small sample sizes. The highest RRs are reached in patients with lower ppoDLCOs. Except in patients with ppoDLCO < 30, the cut-off points where the risk of mortality is 3-fold are below 35 (3), 40 (3,068), and 50 (3,015).
The same procedure was used to analyze total morbidity (respiratory + cardiac morbidity), cardiac morbidity, total respiratory morbidity, and respiratory morbidity that excludes air leak, bronchopleural fistula, and chylothorax.
Comparisons were also made between patients undergoing thoracotomy or VATS with ppoDLCO values between [30–40], [40–50], [50–60], [60–70], [70–80], [80–90], and [90–100]. The main results from these analyses are shown in Fig. 2.
Proportions of the different morbidities obtained after calculating the centered moving average with a range of ±5 values. Gray dots show values 35, 45, 55, 65, 75, 85 and 95. The bars represent the sample size of the ranges [30-40], [40-50], [50-60], [60-70], [70-80], [80-90] and [90-100], with the exact number shown just below. The stars highlight intervals where significant differences are observed between the thoracotomy and VATS patient groups.
Overall morbidity, cardiac morbidity, and respiratory morbidity parameters – after excluding air leak, bronchopleural fistula, and chylothorax – clearly show a divergence in the curves, indicating an increased risk for open surgery.
DiscussionThere is a certain consensus that ppoDLCO is one of the most important measurements in the preoperative study of candidates for major pulmonary resection11, and it is routinely included in most international guidelines2,3. Thus, the decision on whether a patient with resectable lung cancer is a candidate for surgery is based largely on this value. Values below 30% are considered high risk and require a cardiopulmonary exercise test to determine the real risk and to predict the patient’s functional reserve to survive the procedure and overcome possible complications in the postoperative period2. The ACCP clinical practice guideline states that patients with an oxygen consumption of less than 10 ml/kg/min are high risk, and these individuals should be offered alternative surgery (minor resection or minimally invasive surgery) or a non-surgical option2.
Most previous studies to determine the cut-off point of ppoDLCO were conducted on series of patients who did not undergo VATS12,13. More recent studies have shown that in VATS, morbidity and mortality associated with ppoDLCO is lower than in patients undergoing thoracotomy5,7,14. Although some authors maintain that ppoDLCO is a valid predictor for both thoracotomy and VATS15, the same study also reported a significantly greater number of respiratory complications in the thoracotomy group. Thus, we firmly believe that ppoDLCO is a valid predictor for different surgical approaches, but it does seem that the increased risk associated with ppoDLCO differs according to the technique used.
Burt et al.8 published mortality curves and complications associated with ppoDLCO, showing that VATS has a lower morbidity and mortality compared to patients with the same ppoDLCO undergoing open lobectomy. In contrast to our study, cardiac and respiratory morbidity were not separated, but the curves obtained are very similar. While mortality increases linearly in VATS as ppoDLCO decreases, it does so exponentially in thoracotomy.
A recent study by Cao et al.16 on respiratory morbidity associated with ppoDLCO in patients undergoing robot-assisted surgery found curves similar to those of our VATS group, although this study does not compare the technique with any other surgery.
One of the strengths of our study is that it is an analysis of a multicenter, countrywide, prospective registry. We also performed propensity score matching to try to minimize selection bias between the study groups. It can be assumed that the open surgery group will have a higher proportion of complex cases. This constitutes a major selection bias, and makes it unacceptable to directly compare post-operative complications associated with either technique. In our study, several models were created with different degrees of restriction, the most restrictive of which is shown in this report. For this reason, some groups do not show any significant difference, due to the small sample sizes, but we believe that the RR and the curves of the graphs adequately reflect the trend.
It must be said that some trends in the data are not easy to explain. The most notable is the increase in the 90-day mortality rate seen in patients with DLCO values around 80 in the thoracotomy group. No definitive explanation has yet been found, but problems associated with the statistical method can be ruled out, because this observation appeared in all the bias reduction methods tested and is also supported by a considerable sample size (ppoDLCO range [70–80] nVATS = 138 and nOPEN = 161; ppoDLCO range [80–90] nVATS = 91 and nOPEN = 82).
We also need to take the low number of patients with ppoDLCO < 40 to be a limiting factor. This is because the series is surgical and many patients with severely reduced ppoDLCO may have been ruled out for resection surgery since they are considered high risk6,17.
Furthermore, only patients whose ppoDLCO measurement was documented have been evaluated (n = 2,530). This means that 18% of the patients with lung cancer included in the registry do not have DLCO data, either because only ppoFEV1 was determined, or because a cardiopulmonary exercise test had been performed directly.
We must also assume that this is not a randomized clinical trial, so despite the use of score matching to create 2 comparable groups, unevenly distributed variables may persist.
Other limitations that we must assume are the different variables that were not studied that can indirectly affect morbidity and mortality. For example, variables such as intraoperative nodal staging or the application of respiratory physiotherapy are not homogeneous in all participating centers.
ConclusionsVATS lobectomy shows lower morbidity and mortality in patients with the same ppoDLCO. In patients with low ppoDLCO, the risk of 90-day mortality can be up to 3-fold. Furthermore, the steady rise in the risk of mortality begins at higher ppoDLCO values in thoracotomy (∼60) than in VATS (∼45).
It is, therefore, important to adjust functional study values in post-surgical risk assessment in candidates for VATS. Future clinical practice guidelines for the functional study of surgical risk should be adapted to reflect minimally invasive surgery.
FundingAll costs related to the start-up and maintenance of the GEVATS database were covered by Ethicon, Johnson & Johnson. The authors had freedom of research and complete control over the study design, the methods used, the outcome parameters and the results, the analysis of data and the production of the written report.
Conflict of interestsThe authors state that they have no conflict of interests.
We thank all participants in the GEVATS project: Raul Embun, Iñigo Royo-Crespo, José Luis Recuero Díaz, Sergio Bolufer, Sergi Call, Miguel Congregado, David Gómez-de Antonio, Marcelo F. Jimenez, Nicolas Moreno-Mata, Borja Aguinagalde, Sergio Amor-Alonso, Miguel Jesús Arrarás, Ana Isabel Blanco Orozco, Marc Boada, Alberto Cabañero Sánchez, Isabel Cal Vázquez, Ángel Cilleruelo Ramos, Silvana Crowley Carrasco, Elena Fernández-Martín, Santiago García-Barajas, Maria Dolores García-Jiménez, Jose María García-Prim, Jose Alberto Garcia-Salcedo, Juan José Gelbenzu-Zazpe, Carlos Fernando Giraldo-Ospina, María Teresa Gómez Hernández, Jorge Hernández, Jennifer D. Illana Wolf, Alberto Jauregui Abularach, Unai Jiménez, Iker López Sanz, Néstor J. Martínez-Hernández, Elisabeth Martínez-Téllez, Lucía Milla Collado, Roberto Mongil Poce, Francisco Javier Moradiellos-Díez, Ramón Moreno-Balsalobre, Sergio B. Moreno Merino, Carme Obiols, Florencio Quero-Valenzuela, María Elena Ramírez-Gil, Ricard Ramos-Izquierdo, Eduardo Rivo, Alberto Rodríguez-Fuster, Rafael Rojo-Marcos, David Sanchez-Lorente, Laura Sanchez Moreno, Carlos Simón, Juan Carlos Trujillo-Reyes, Florentino Hernando Trancho, Cipriano López, Juan José Fibla, Julio Sesma.
We thank Johnson & Johnson for their collaboration in the development of the Spanish VATS Group. We also thank all the clinical documentation managers at each hospital for actively participating in the audit of our study.
Please cite this article as: Aguinagalde B, Insausti A, Lopez I, Sanchez L, Bolufer S, Embun R. La lobectomía VATS tiene una menor morbimortalidad para una misma ppoDLCO: análisis de base de datos del Grupo Español de Cirugía Torácica Video-asistida. Arch Bronconeumol. 2021. https://doi.org/10.1016/j.arbres.2021.01.030







![Proportions of the different morbidities obtained after calculating the centered moving average with a range of ±5 values. Gray dots show values 35, 45, 55, 65, 75, 85 and 95. The bars represent the sample size of the ranges [30-40], [40-50], [50-60], [60-70], [70-80], [80-90] and [90-100], with the exact number shown just below. The stars highlight intervals where significant differences are observed between the thoracotomy and VATS patient groups.](https://static.elsevier.es/multimedia/15792129/0000005700000012/v3_202201080532/S1579212921003797/v3_202201080532/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w98FxLWLw1xoW2PaQDYY7RZU=)








