New evidence and knowledge about the clinical management of drug-resistant tuberculosis (TB) in the last 3 years, makes it necessary to update the recent guideline published by SEPAR in 2017, mainly in relation to new diagnostic methods, drug classification, and regimens of treatment recommended to treat patients with isoniazid-resistance TB, rifampicin resistance TB and multidrug-resistant TB. With respect to tuberculosis diagnosis, we recommend the use of rapid molecular assays that also help to detect mutations associated with resistance. In relation to the treatment of multidrug-resistant TB we prioritize effective all-oral shorter treatment regimens including bedaquiline, a fluoroquinolone (levofloxacin or moxifloxacin), bedaquiline and linezolid, instead of the previously recommended short-course treatment with aminoglycosides and other less effective and more toxic drugs. The design of these regimens (initial schedule and changes in the regimen if necessary) should be made in accordance with drug-resistant TB experts; the treatment should be the responsibility of personnel with experience in the treatment of TB and in TB units guaranteeing the follow-up of the treatment and the management of drugs adverse effects.
La evidencia acumulada en los 3 últimos años sobre el manejo clínico de la tuberculosis (TB) con resistencia a fármacos ha sido tan importante que hace necesario actualizar la normativa que SEPAR publicó en 2017, sobre todo en lo referente a nuevos métodos diagnósticos, a la clasificación racional de los fármacos con actividad frente a Mycobacterium tuberculosis y a los esquemas básicos a recomendar en los pacientes con TB con resistencia a isoniacida, con resistencia a rifampicina o con multifarmacorresistencia. En el diagnóstico de la enfermedad recomendamos la utilización de métodos moleculares rápidos que son útiles además para la detección precoz de mutaciones asociadas a resistencias a fármacos. Para el tratamiento de los enfermos con TB con multifarmacorresistencia se hace necesario dar prioridad a esquemas orales acortados que incluyan bedaquilina, una fluoroquinolona (levofloxacino o moxifloxacino) y linezolid en lugar de los esquemas cortos previamente recomendados con aminoglucósidos y otros muchos fármacos de menor eficacia y más tóxicos. La recomendación de la normativa es que el diseño de los tratamientos en estos pacientes, tanto el inicial como si se precisan cambios, sea consultado siempre con expertos en el manejo de TB con resistencia a fármacos y que se realicen por personal con experiencia en TB y en unidades que garanticen la supervisión de los tratamientos y el abordaje de sus reacciones adversas.
In 2017, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) published guidelines on the diagnosis and treatment of drug-resistant tuberculosis (DR-TB).1 Since important updated evidence on the management of these patients has appeared,2–4 prompting the publication of 2 new guidelines by the World Health Organization (WHO),5,6 and another by the American Thoracic Society (ATS)/Centers for Disease Control and Prevention (CDC)/European Respiratory Society (ERS)/Infectious Diseases Society of America (IDSA),7 in addition to recent WHO communications on TB diagnosis and treatment.8–10
In the light of this new knowledge, an update of the 2017 SEPAR guidelines is needed.
Diagnosis of TuberculosisXpert® MTB/RIF UltraCepheid has developed a new generation Xpert® MTB/RIF Ultra technique with enhanced susceptibility using 2 amplification targets (IS6110 and IS1081) and a larger polymerase chain reaction chamber (50μl in Ultra, vs 25μl in the Xpert® MTB/RIF). The new Ultra system features a lower mycobacterial detection limit (16 colony-forming units per ml compared to 131 in the Xpert® MTB/RIF).11,12
It uses the same semi-quantitative categories as Xpert® MTB/RIF (high, medium, low and very low), but a new category called “detected trace” has been added, offering increased sensitivity (90% sensitivity in pulmonary TB compared to sputum culture).8 In people living with human immunodeficiency virus (HIV), children, and patients being evaluated for extrapulmonary TB or suspected TB, “trace calls” should be considered as true positives.11
The current WHO recommendation for the use of Xpert® MTB/RIF also applies to the Ultra technique: it should be used as the initial diagnostic test in all adults and children with signs and symptoms of TB and for the study of selected extrapulmonary samples (cerebrospinal fluid, lymph nodes and tissue samples), and samples from children (nasopharyngeal, gastric, and stool specimens8,11).
Possibility of Using Other Rapid Molecular Methods for the Diagnosis of Tuberculosis and Drug-resistant TuberculosisSince 2017, evidence has accumulated to recommend other molecular methods, either to replace Xpert® MTB/RIF (normal or Ultra version) or to supplement it, since this test only detects rpoB mutations. Some of the different available alternatives include: BD MAX® MDR-TB (Becton Dickinson)13; Abbott RealTime® MTB RIF/INH assay; FluoroType® MTBDR (Hain); Anyplex® MTB/NTM y Anyplex® MTB/MDR/XDR (Seegene); TrueNat® (Molbio Diagnostics).14 These techniques all use real-time polymerase chain reaction amplification systems for specific targets, with varying degrees of automation. In addition to detecting Mycobacterium tuberculosis complex and rifampicin resistance (RR), some can be used (in 1 or 2 steps) to amplify the spectrum of isoniazid (H) resistance detection (BD MAX® MDR-TB, Abbott RealTime® MTB RIF/INH assay, FluoroType® MTBDR, Anyplex® MTB/MDR/XDR). Anyplex® MTB/NTM can simultaneously detect non-tuberculous mycobacteria, information that is highly useful in differential diagnosis when the diagnosis of M. tuberculosis disease is not confirmed by any of the techniques used. They can also be used to amplify resistance detected to fluoroquinolones (FQ) and aminoglycosides/polypeptides (Anyplex® MTB/MR/XDR), the latter being similar to the GenoType®MDRsl already mentioned in our 2017 recommendation.
We will clearly have to remain alert to the development of new technologies in the immediate future, given the advances in the diagnosis of this disease.
Finally, it should be noted that molecular tests can also help improve the final outcome of patients with TB (the use of Xpert® MTB/RIF as an initial test to replace sputum smears has led to improved cure rates, reduced mortality and fewer cases lost before start of treatment8).
Discrepancies in Rifampicin Resistance Results by Different MethodsIn line with current evidence, it is agreed that a patient who shows TB with RR (RR-TB) using any properly performed method (either phenotypic or molecular) should be considered and treated as such, even if the results from other methods are conflicting.15 Complete genome sequencing of M. tuberculosis helps resolve discrepancies and allows for the detection of mutations not identified by other methods.16,17 It is a test that will certainly become part of routine diagnosis as its use becomes widespread.
Basis for Treatment of all Forms of Tuberculosis, Both Susceptible and Drug-ResistantNumber of Drugs Needed to Treat tuberculosisThe recommendation to use at least 4 previously unused drugs, or drugs with proven M. tuberculosis susceptibility, was made on the basis that there might be resistance to one of the 4 compounds (as in the case of H in the initial regimen) and because some of them might have reduced efficacy, such as the case of ethionamide (Eto)/prothionamide (Pto), cycloserine, or para-aminosalicylic acid in patients with RR-TB or multidrug-resistant (MDR)-TB (TB resistant to at least H+R).18,19 Susceptibility to H+R can now be determined at the beginning of treatment, and powerful drugs, such as FQ, linezolid (LZD), bedaquiline (BDQ) and clofazimine (Cfz), that have a very low probability of being resistant (a rapid molecular test can be performed in the case of FQ) and show good bactericidal and sterilizing activity,18–21 can be used in the initial regimen. Thus, a course of only 3 new drugs for 6–9 months may be sufficient to cure TB with a minimum risk of acquisition of resistance or subsequent relapses.18–20
If susceptibility results are not available for any of the key drugs for which reliable susceptibility tests are available (H, R, FQ), or if such tests do not exist (as in the case of BDQ), doubts regarding resistance may arise and recourse to some drugs of doubtful efficacy may be necessary. In such cases, the recommendation of at least 4 drugs to treat TB would remain in force.18,20,22 In the case of BDQ, some publications have warned of the emergence of resistance,23,24 but these cases remain exceptional and are limited to settings in which the drug has not been used properly. We must remain alert to these communications, but for the moment, this drug can be expected to be susceptible in Spain.
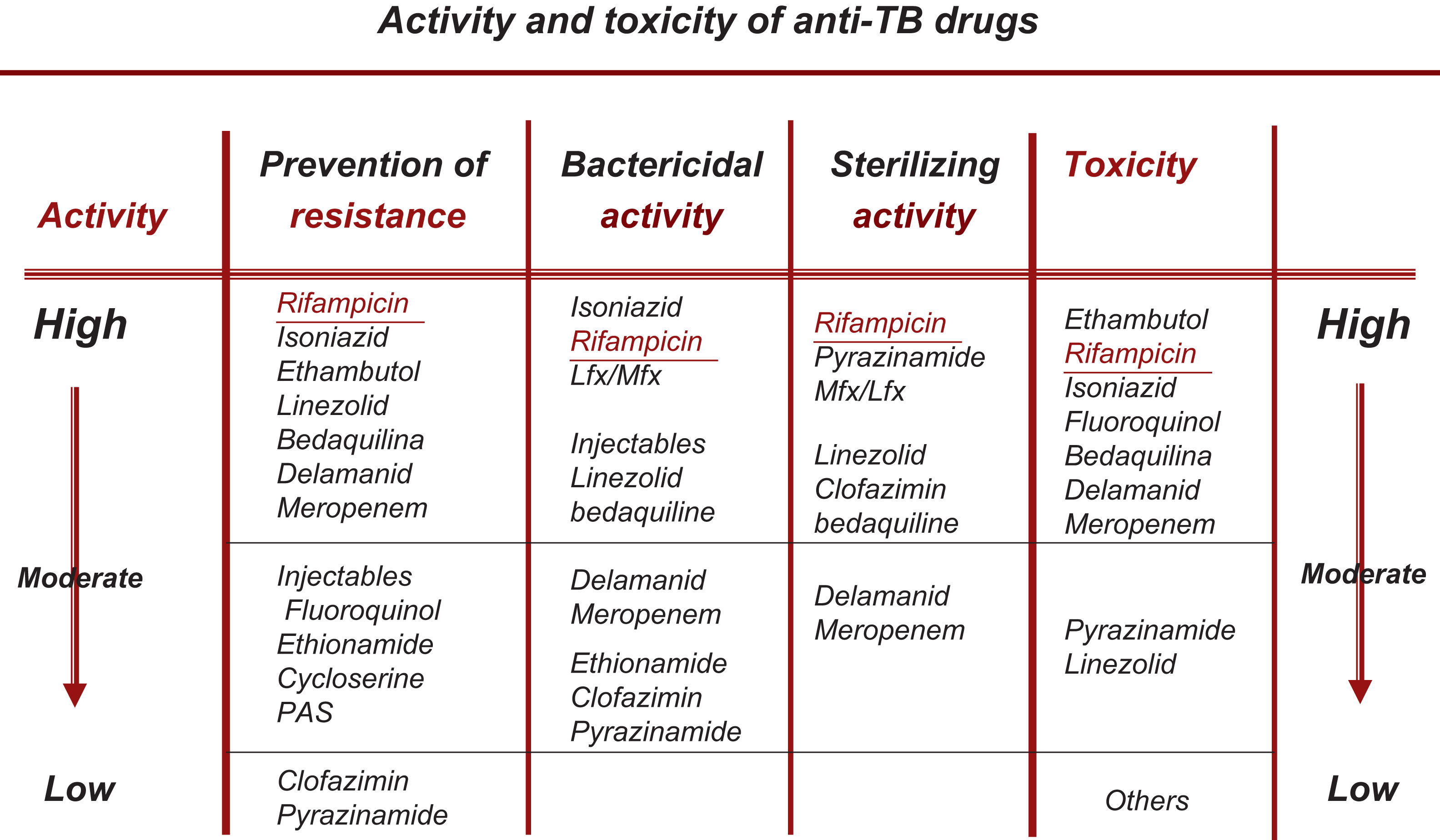
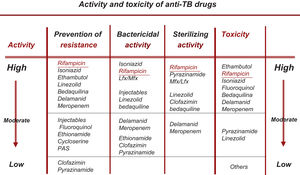
Change in the Choice of So-called Essential and Accompanying DrugsAll the new drugs (FQ, Lzd, BDQ, Cfz) that are already fully incorporated into the treatment of RR-TB/MDR-TB can be considered essential (with good bactericidal and/or sterilizing capacity), so the use of accompanying medications would be unnecessary, except in situations of widespread resistance.19,20 At present, the recommendation should be to use at least 3 essential drugs, with at least 1–2 that show good bactericidal activity and 1–2 with good sterilizing activity. No accompanying drug should be included unless unavoidable22 (Fig. 1).
Rational Classification of Drugs With Activity Against M. tuberculosisThis section has been substantially modified based on the results of the WHO meta-analysis, on changes in the classification subsequently recommended by the WHO and on updated data on the bactericidal and/or sterilizing activity of these drugs as set out in Table 1.3,6,21
Rational Classification and Sequential Use of Anti-tuberculosis Drugs in the Design of a Treatment Regimen for Both Drug-susceptible and Drug-resistant Tuberculosis. (This Table Updates Table 3 of the 2017 Guidelines1).
| Group 1. First-line oral drugs |
| - Essential: Rifampicin, isoniazid and pyrazinamide |
| - Accompanying: Ethambutol |
| Group 2. This corresponds to the current WHO group A.6Three groups of drugs are included here, to be prioritized in the following order: |
| a Levofloxacin or moxifloxacin. Ideally, resistance to these drugs should be ruled out using rapid molecular methods such as GenoType® or Anyplex® |
| b Linezolid |
| c Bedaquiline |
| Group 3. This corresponds to the current WHO group B.6Two drugs are included here, one of which (clofazimin) has much greater evidence of action than the other (cycloserin). If one of the 2 is to be chosen, clofazimin will always be givenpriority3,6 |
| a Clofazimin. This should be the drug of choice if any of the drugs in group 2 cannot be used |
| b Cycloserine. In some specific cases, some of the drugs in group 4 may be used prior to cycloserine on the basis of their better bactericidal and/or sterilizing action |
| Group 4. This corresponds to the current WHO group C,6but the sequence of inclusion in the regimens should be asfollows20: |
| a Meropenem, or imipenem/cilastatin. Both should be given at the same time as amoxicillin/clavulanate to facilitate their effectiveness |
| b Delamanid. Sometimes it may be preferable to use this drug before carbapenems because of the possibility of oral administration |
| c Amikacina. This should only be used if these 3 conditions are met. |
| 1 Possible resistance has been ruled out by a rapid molecular test |
| 2 Periodic audiometric checks can be done |
| 3 There are no other drugs available among those previously listed in groups 2 and 3 |
| d Ethionamide or prothionamide. |
| e Pyrazinamide. Its use here applies to cases of rifampicin-resistant tuberculosis/multidrug-resistant tuberculosis |
| f Ethambutol. Its use here applies to cases of rifampicin-resistant tuberculosis/multidrug-resistant tuberculosis |
| g Para-aminosalicylic acid |
Important changes have been made, especially in the case of RR-TB/MDR-TB and extensively drug-resistant TB (XDR-TB, meaning MDR-TB plus resistance to at least one FQ and to a second-line injectable drug [kanamycin, amikacin, capreomycin]) listed in Tables 2 and 3.
Recommended Basic Regimens for Patients With Susceptible Tuberculosis and Mono/Polyresistance.1,20,22 (This Table Updates Table 4 of the 2017 Guidelines).
| 1. Initial TB cases with R susceptibility (known or unknown Hsusceptibilitya,b) |
| - 2 HRZE/4 HR, or 2 HRZ/4 HR |
| * 2 HRZ/4 HR in cases where susceptibility to H can be determined in the first 2 weeks |
| 2. TB cases with resistance to H (mono- or polyresistance), but with susceptibility toRc,d |
| - 9 HRZE, or 6 LFx-RZE (H) |
| 3. Cases with resistance to R (mono- or polyresistance), but with susceptibility to H, or if susceptibility to H is not known |
| - Same treatment as MDR-TB, which is discussed in Table 4, adding H to the regimen, but not taking into account it among the 4 new drugs |
E: ethambutol; H: isoniazid; LFx: levofloxacin; R: rifampicin; TB: tuberculosis; Z: pyrazinamide.
Do not switch to the continuation phase (4HR) until one of the following 2 circumstances occurs: sputum smear is already negative, or that susceptibility to H and R is determined.
Treatment should be prolonged beyond 6 months in patients in whom sputum smear and/or culture conversion is delayed beyond 2 months.1,18 As a reference, these patients will receive prolonged treatment with H+R up to a minimum of 4 months after the cultures are negative.
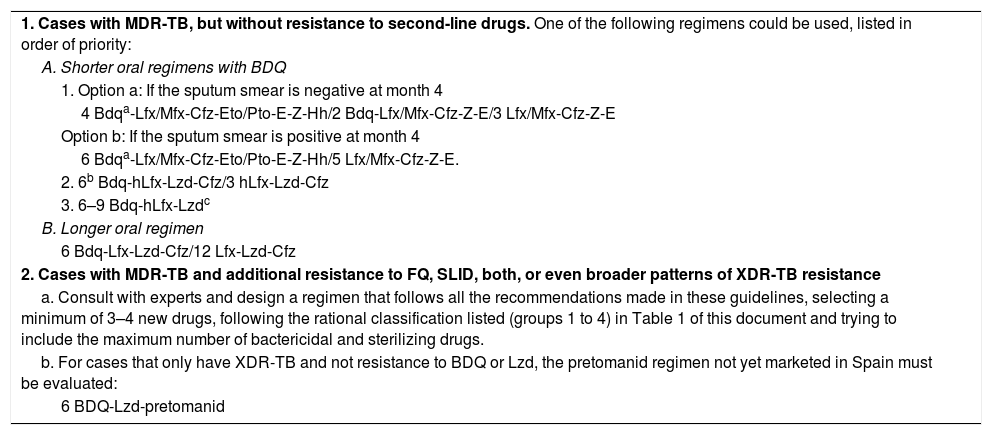
Recommended Basic Regimens for Patients With MDR-TB.6,9,10,19,20,22,30–32 (This Table Updates Table 5 of the 2017 Guidelines).
| 1. Cases with MDR-TB, but without resistance to second-line drugs. One of the following regimens could be used, listed in order of priority: |
| A. Shorter oral regimens with BDQ |
| 1. Option a: If the sputum smear is negative at month 4 |
| 4 Bdqa-Lfx/Mfx-Cfz-Eto/Pto-E-Z-Hh/2 Bdq-Lfx/Mfx-Cfz-Z-E/3 Lfx/Mfx-Cfz-Z-E |
| Option b: If the sputum smear is positive at month 4 |
| 6 Bdqa-Lfx/Mfx-Cfz-Eto/Pto-E-Z-Hh/5 Lfx/Mfx-Cfz-Z-E. |
| 2. 6b Bdq-hLfx-Lzd-Cfz/3 hLfx-Lzd-Cfz |
| 3. 6–9 Bdq-hLfx-Lzdc |
| B. Longer oral regimen |
| 6 Bdq-Lfx-Lzd-Cfz/12 Lfx-Lzd-Cfz |
| 2. Cases with MDR-TB and additional resistance to FQ, SLID, both, or even broader patterns of XDR-TB resistance |
| a. Consult with experts and design a regimen that follows all the recommendations made in these guidelines, selecting a minimum of 3–4 new drugs, following the rational classification listed (groups 1 to 4) in Table 1 of this document and trying to include the maximum number of bactericidal and sterilizing drugs. |
| b. For cases that only have XDR-TB and not resistance to BDQ or Lzd, the pretomanid regimen not yet marketed in Spain must be evaluated: |
| 6 BDQ-Lzd-pretomanid |
BDQ: Bedaquiline; Cfz: clofazimine; E: ethambutol; Eto: ethionamide; FQ: fluoroquinolones; hH: high doses of H (15–20mg/kg weight); hLfx: high doses of LFx (1000mg/day); Lfx: levofloxacin; Lzd: linezolid; MDR-TB: multidrug-resistant tuberculosis; MFX: Moxifloxacin; Pto: protionamide; SLID: second-line injectable drugs; XDR-TB: extensively multidrug-resistant TB; Z: pyrazinamide.
If sputum smear remains positive at the end of month 4, this intensive phase should be prolonged up to 6 months with all of the same drugs (option b). If still positive at 6 months, this should be taken as an indicator that the regimen is failing and an alternative regimen should be considered.
There are no changes with respect to the previous guidelines. In new cases of TB in which susceptibility to all drugs is presumed, or if the absence of mutations in the rpoB gene (molecular detection of resistance to R) has been confirmed by molecular testing, the ideal treatment regimen is 2 HRZE/4 HR. However, if the absence of gene mutations in rpoB, katG and inhA (molecular detection of resistance to H) is confirmed by molecular testing in the first days of treatment, 2 HRZ/4 HR would be sufficient14,18(strong recommendation, high quality of evidence [⊕⊕⊕⊕]).
To reduce the possibility of errors and the possible selection of resistance, these drugs should always be administered in fixed combination doses and with directly observed treatment in patients who have risk factors for poor therapeutic compliance.1
Treatment of Tuberculosis Resistant to Isoniazid (Mono- or Polyresistance), But Susceptible to RifampicinThe 9 HRZE regimen recommended as a priority in the 2017 guidelines should still be prioritized1(conditional recommendation, low [⊕] to very low [] evidence quality). The use of high doses of H may be evaluated, especially if a rapid molecular test (GenoType®) that shows the absence of mutation in the katG gene is available. However, the other regimens recommended in our 2017 guidelines, consisting of 2 FQ-REZ/7 FQ-RE (E: ethambutol, Z: pyrazinamide), should be replaced by the WHO recommended regimen of 6 Lfx-REZ(H) (Lfx: levofloxacin),5,6 while taking into account our previous recommendations for the FQ-containing regimen, i.e., Lfx should only be included in the regimen if it is administered from the beginning with the other drugs. It should not be added if H resistance results will only be received after 3–4 weeks of treatment, due to the possible risk of inadvertent monotherapy. These recommendations, which led to the inclusion of LFx in this regimen, are based on a meta-analysis2 in which the vast majority of patients came from sites where the results of H resistance testing were determined very quickly, but this situation is unusual, even in our setting. It should also be noted that another meta-analysis25 found that 9 RZE is equally effective in curing cases with resistance or susceptibility to H.
Treatment of Tuberculosis Resistant to Rifampicin (Mono- or Polyresistance), But Susceptible to IsoniazidIt is still true to say that cases of isolated resistance to R are rare in clinical practice, and that since resistance to R determines prognosis in patients with MDR-TB, these patients should be managed as MDR-TB patients, and treated as such, adding H to the regimen, of course, because if its susceptibility is confirmed, it will be an important contribution to treatment1,6(conditional recommendation, low [⊕] to very low [] quality of evidence).
Treatment of Multidrug-Resistant Tuberculosis (MDR-TB)The significant amount of evidence accumulated in this section3,4,6,19,20 requires a significant change in the recommendations made in the 2017 guidelines.1 Fortunately, this evidence is bringing about almost continuous changes in recent months. Thus, in March 2019, the WHO guidelines6 prioritized long individualized oral regimens of 18–20 months’ duration. However, after studies with new drugs appeared, the WHO itself published a rapid communication in December 20199 recommending that priority be given to shorter oral regimens including BDQ, and in a new publication that appeared in January 2020,10 based on accumulated evidence, it recommended prioritizing a shorter BDQ regimen.
Therefore, based on the latest evidence in the treatment of RR-TB/MDR-TB, priority should be given to shorter oral regimens based on BDQ,9,10 and shorter (or longer) injectable regimens should no longer be used in RR-TB/MDR-TB As a result, the regimen that was recommended as a priority in our 2017 guidelines should no longer be used, especially as the cumulative evidence has shown that oral regimens are better and much less toxic.
Based on this, one of the following regimens may be recommended in these patients:
- (A)
Shorter oral regimens with BDQ
In this section, one of the following 3 regimens could be considered. The advantages and disadvantages of these regimens will be analyzed.
1. This first regimen offers 2 possibilities:
- (a)
If the sputum smear is negative at month 4: 4 Bdq*-Lfx/moxifloxacin (Mfx)-Cfz-Eto/Pto-E-Z-hH/2 BDQ-Lfx/Mfx-Cfz-Z-E/3 Lfx/Mfx-Cfz-Z-E.
- (b)
If the sputum smear is positive at month 4: 6 BDQ*-Lfx/Mfx-Cfz-Eto/Pto-E-Z-hH/5 Lfx/Mfx-Cfz-Z-E.(conditional recommendation, low [⊕] to very low [] quality of evidence)
*In both options, BDQ should be administered for 6 months, accompanied in the first option by intensive-phase drugs for the first 4 months and continuation-phase drugs for the next 2 months, completing treatment with 3 more months of continuation-phase drugs alone (9 months total). However, if the sputum smear remains positive at the end of month 4, all drugs in this intensive phase and BDQ will be administered for 6 months, followed by continuation-phase drugs for 5 months, for a total duration of treatment of 11 months (option b). If the sputum smear remains positive at the end of month 6, this regimen will be considered to have failed and a different regimen should be designed. hH means high-dose H.
This treatment regimen has the advantage that it has the most supporting evidence, and is therefore recommended as a priority by the WHO in its latest publication in January 2020.10 It is practically the same as the regimen we recommended in our 2017 guidelines,1 and it is supported by a meta-analysis26 and a randomized clinical trial,27 the only difference being the use of BDQ instead of amikacin. However, it has the drawback that it still uses 7 drugs in the intensive phase, including some with very little or doubtful efficacy,3 such as Eto/Pto, E, Z and hH; some of which are as poorly tolerated as Eto/Pto.
Because of the speed with which new evidence is accumulating on this topic, the WHO will probably soon amend its recommendations in favor of one of the other 2 options set out below.
2. 6 BDQ-hLfx-LZD-Cfz/3 hLfx-LZD-Cfz (conditional recommendation, low [⊕] to very low [] quality of evidence)
If the sputum smear remains positive at the end of month 6, this regimen will be considered to have failed and a different individualized regimen should be designed. (hLfx means high-dose Lfx). hLfx is preferred because it causes less QTc interval prolongation on electrocardiogram than Mfx,28 taking into account that the regimen contains 2 other drugs that also prolong QTc (BDQ and Cfz).
This regimen has the advantage that it uses the 4 drugs that are given priority in the WHO recommendations of 20196 and, since they all have sterilizing activity,21 a total of 9 months of treatment would be sufficient. Moreover, since BDQ, Lzd, and Cfz have been used sparingly in the treatment of RR-TB/MDR-TB in Spain, drug susceptibility may be assumed to be highly probable.
Ideally, a susceptibility test should be performed to rule out resistance to FQ before beginning this regimen, although it could also apply to patients with RR-TB/MDR-TB who have never received these drugs for TB treatment.
3. 6–9 BDQ-hLfx-Lzd (conditional recommendation, low [⊕] to very low [] quality of evidence)
According to the section on the number of drugs needed to treat TB, a 6-month regimen with high doses of LFx+Lzd+BDQ18,19 would meet all requirements to be considered an effective regimen. It consists of 3 new drugs, all of which have bactericidal and sterilizing capacity, although susceptibility must be confirmed for Lfx.18–21 In this regimen, LFx is preferred to MFX, because it is causes less QTc prolongation on electrocardiogram,28 taking into account that the regimen already contains another drug with the same effect (BDQ). If the sputum smear is still positive at the end of month 2, the regimen should be prolonged until 9 months, provided that the sputum smear and culture are negative at the end of month 4 of treatment.15
This regimen is very similar to the BPaL combination being successfully tested in the NIX-TB randomized clinical trials in patients with XDR-TB.29,30 The 6-month of Lzd+BDQ+pretomanid has been approved by the U.S. FDA.31 Promising outcomes have been published recently32 and it has already been included in the WHO recommendations.9 Pretomanid is not marketed in Spain. The study30,32 has limitations that were mentioned in the recent WHO communication: it has a small number of patients (108) and adverse effects were observed (hematological, hepatic, optical and peripheral neuropathy), although a large proportion of these were related to the use of high doses of Lzd (1200mg/day), whereas 600mg/day may be sufficient. The shorter oral regimen proposed in these guidelines would only lead, in terms of NIX-TB to switching LFx for pretomanid because BPaL was indicated for patients with FQ resistance. Moreover, according to the available data, pretomanid is no better than Lfx in the treatment of TB.33
The advantages of these 6–9-month oral regimens (Lfx+Lzd+BDQ in MDR-TB and Lzd+BDQ+pretomanid in XDR-TB) are that all drugs are administered orally, they use the best second-line drugs, they are not ototoxic, they do not need ion monitoring, and because the courses are much shorter, the potential risk of dropout is reduced. The drawbacks of these regimens are similar to the others, including the need to monitor the QTc interval (when they contain 2 drugs – LFx and BDQ – that prolong QTc) and to monitor the possible toxicity of Lzd, although this should be less than in the other regimens as the time of administration is shorter.
A regimen such as the one discussed, which is shorter and administered orally, must be used in programmatic research conditions (patient monitoring, support, and proper inclusion, principles of good clinical practice, informed patient consent, active monitoring and treatment of drug side effects, treatment monitoring, evaluation of the final outcome, standardized data collection), which would constitute the minimum requirements for the management of these patients.9
It is therefore very possible that patients will immediately be able to receive these novel treatments under the conditions described above.9
One important limitation associated with BDQ is its high price and difficult accessibility. Given its essential role in the new treatment regimens, efforts must be made at the institutional level to facilitate its availability in Spain.
- (B)
Longer oral regimen
Although this regimen was recommended until a few months ago as a priority by WHO,6 it should now be relegated to a second step,10,11 which would consist of a 6-month intensive phase with BDQ+Lfx/Mfx+Lzd+Cfz, plus a 12-month continuation phase with Lfx/Mfx+Lzd+Cfz (conditional recommendation, low [⊕] to very low [] evidence quality), thus meeting the criteria of the WHO recommendations of March 2019.6 The regimen proposed by ATS/CDC/ERS/IDSA7 follows the same order of priority and drug groups as that of WHO, although it differs in terms of higher drug numbers (5 in the intensive phase and 4 in the continuation phase) and a longer treatment time (15 to 21 months after culture conversion).7
There is no doubt that this regimen will be very effective, because it uses the best drugs available in the treatment of RR-TB/MDR-TB.3,6 However, based on the above considerations, it may involve too many drugs (4) administered for too long, especially since they are all very potent with almost certain susceptibility and sterilizing capacity.19,20,34
The disparities between these international guidelines6,7 may emerge from the fact that the recommendations have a low or very low quality of evidence. Moreover, both guidelines are based on a meta-analysis performed by the WHO itself3 to assess the value of each drug in the different RR-TB/MDR-TB regimens. This study concluded that Lzd, carbapenems, Lfx/Mfx, BDQ and Cfz were the most effective drugs, and the remaining drugs contributed little to the possible success of RR-TB/MDR-TB treatment; the latter included Eto/Pto, cycloserine, para-aminosalicylic acid, E, and Z. It also found that the inclusion of drugs such as kanamycin or capreomycin was associated with a worse therapeutic outcome. This meta-analysis3 also concluded that the most effective regimen was a combination of 4–5 effective drugs and a minimum duration of 18 months. However, a recognized limitation of this study is that practically all the regimens analyzed contained quite a few drugs associated with very slight or no improvement in outcomes (ineffective, weak drugs),3 and few had useful sterilizing activity.19
Treatment of Patients With Extensively Drug-Resistant Tuberculosis (XDR-TB) or Even Broader Resistance PatternsWhile significant progress has also been made in this area, we continue to believe that these forms of TB are so difficult to manage (in clinical practice and in programmatic conditions) that they should be treated by highly skilled specialists and in units that can guarantee close supervision of the treatment and proper management of adverse reactions.
Contact TracingThe news in this area is that enough evidence has been accumulated to be able to recommend, as an alternative to the periodic reviews that remain the most widely accepted recommendation, a preventive regimen with an FQ (Mfx or LFx)35 in close contacts of RR-TB/MDR-TB cases in whom disease is ruled out, especially in children or immunosuppressed individuals. This treatment should be administered for a period of 6 months (conditional recommendation, very low [] quality of evidence).
Treatment of Tuberculosis in People Living With Human Immunodeficiency VirusTreatment of drug-susceptible or drug-resistant TB in HIV-infected patients is the same as in non-infected patients. In patients with both infections who have not started treatment, starting TB treatment will be prioritized. If the patient has CD4 <50mm–3 at 2 weeks, good adherence, and no side effects have been confirmed, combination antiretroviral therapy (cART) will be started. In patients with higher CD4 counts, cART can begin after month 2, when the patient is already taking fewer TB drugs. Better survival has been observed with these regimens.36,37 In cases of tuberculous meningitis, cART should start after 8 weeks of TB treatment.38
The interactions between rifamycins and some antiretrovirals should be taken into account; in these cases, rifabutin may be an alternative to rifampicin but that would rule out fixed drug doses, so a cART based on efavirenz would be advisable.39
These patients should be managed by clinical experts in both infections, with monitoring for adherence to both treatments (using directly observed treatment if necessary; methadone maintenance programs help treatment in heroin users). Side effects and the possibility of immune reconstitution syndrome should be monitored, along with the usual follow-up.39 TB-HIV co-infection is a very serious worldwide problem that will only be solved with firm political commitment.40
Conclusions- 1.
The systematic use of rapid molecular tests is recommended in the diagnosis of TB to increase the diagnostic sensitivity of the disease, to help early detection of drug resistance, and to achieve better therapeutic outcomes for patients.
- 2.
Although resistance in TB complicates treatment and the chances of success, if basic management guidelines are followed, acceptable cure rates can be achieved in the vast majority of patients. These basic procedures, which summarize virtually the entire guidelines, are set out in Table 4.
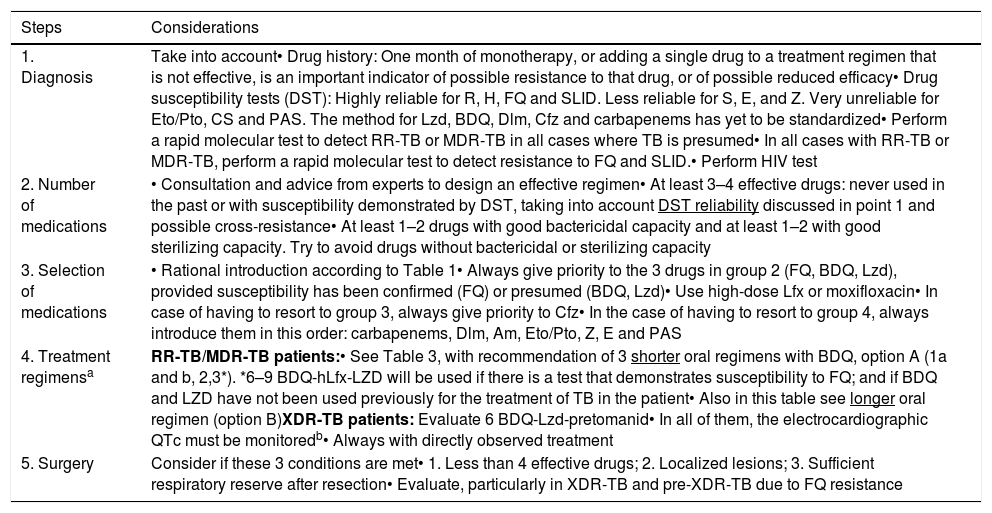
Table 4.Summary of Good Practices in MDR-TB Management. This Table Updates Table 8 of the 2017 guidelines.1
Steps Considerations 1. Diagnosis Take into account• Drug history: One month of monotherapy, or adding a single drug to a treatment regimen that is not effective, is an important indicator of possible resistance to that drug, or of possible reduced efficacy• Drug susceptibility tests (DST): Highly reliable for R, H, FQ and SLID. Less reliable for S, E, and Z. Very unreliable for Eto/Pto, CS and PAS. The method for Lzd, BDQ, Dlm, Cfz and carbapenems has yet to be standardized• Perform a rapid molecular test to detect RR-TB or MDR-TB in all cases where TB is presumed• In all cases with RR-TB or MDR-TB, perform a rapid molecular test to detect resistance to FQ and SLID.• Perform HIV test 2. Number of medications • Consultation and advice from experts to design an effective regimen• At least 3–4 effective drugs: never used in the past or with susceptibility demonstrated by DST, taking into account DST reliability discussed in point 1 and possible cross-resistance• At least 1–2 drugs with good bactericidal capacity and at least 1–2 with good sterilizing capacity. Try to avoid drugs without bactericidal or sterilizing capacity 3. Selection of medications • Rational introduction according to Table 1• Always give priority to the 3 drugs in group 2 (FQ, BDQ, Lzd), provided susceptibility has been confirmed (FQ) or presumed (BDQ, Lzd)• Use high-dose Lfx or moxifloxacin• In case of having to resort to group 3, always give priority to Cfz• In the case of having to resort to group 4, always introduce them in this order: carbapenems, Dlm, Am, Eto/Pto, Z, E and PAS 4. Treatment regimensa RR-TB/MDR-TB patients:• See Table 3, with recommendation of 3 shorter oral regimens with BDQ, option A (1a and b, 2,3*). *6–9 BDQ-hLfx-LZD will be used if there is a test that demonstrates susceptibility to FQ; and if BDQ and LZD have not been used previously for the treatment of TB in the patient• Also in this table see longer oral regimen (option B)XDR-TB patients: Evaluate 6 BDQ-Lzd-pretomanid• In all of them, the electrocardiographic QTc must be monitoredb• Always with directly observed treatment 5. Surgery Consider if these 3 conditions are met• 1. Less than 4 effective drugs; 2. Localized lesions; 3. Sufficient respiratory reserve after resection• Evaluate, particularly in XDR-TB and pre-XDR-TB due to FQ resistance Am: amikacin; BDQ: bedaquiline; Cfz: clofazimine; Cs: cycloserine; DLM: delamanid; DST: drug susceptibility test; E: ethambutol; Eto: ethionamide; FQ: fluoroquinolones; H: isoniazid; HIV: human immunodeficiency virus; hLfx: high doses of Lfx (1000mg/day): Lfx: levofloxacin; Lzd: linezolid; MDR-TB: multidrug-resistant tuberculosis; PAS: para-aminosalicylic acid; Pto: protionamide; R: rifampicin; S: streptomycin; RR-TB: rifampicin-resistant tuberculosis; SLID: second-line injectable drugs; XDR-TB: extremely drug-resistant tuberculosis; Z: pyrazinamide.
bLevofloxacin, moxifloxacin and clofazimin, like bedaquiline and delamanid, may cause QTc alterations on ECG. We recommend two papers42,43 on the effects of drugs used in the treatment of MDR and XDR tuberculosis on QTc on ECG.
- 3.
Treatment plans for these patients, both initial and adjusted, should always be consulted with experts. To this end, health authorities41 and/or scientific societies should promote the organization of expert groups at state level.
- 4.
The top priorities will continue to be to offer adequate treatment to all patients with susceptible TB and to achieve good adherence in order to avoid the development of resistance.
Please cite this article as: Caminero JA, García-García J-M, Caylà JA, García-Pérez FJ, Palacios JJ, Ruiz-Manzano J. Actualización de la normativa SEPAR «Diagnóstico y tratamiento de la tuberculosis con resistencia a fármacos». Arch Bronconeumol. 2020;56:514–521.