Malignant pleural effusion (MPE) is conventionally treated with the administration of intrapleural talc, causing an inflammatory reaction that fuses the pleural layers, avoiding the production of pleural fluid (PF). As an alternative approach, we decided to administer intrapleural chemotherapy using a permanent tunneled catheter (PTC) with the aim of repeating the treatment in different cycles.
Our first case was a 65-year-old woman with a diagnosis of diffuse large B-cell non-Hodgkin's lymphoma, stage IVA, with pleural infiltration, MPE, and a mass in the right posterior chest wall measuring 15×10×4cm, infiltrating the 8th–10th rib and chest wall.
In the first 2 cycles of immunochemotherapy with dose-adjusted EPOCH-rituximab, pleural effusion (PE) was managed with drainage by thoracocentesis. Before the third cycle, the patient developed massive PE, so we decided to use Pleurx®. After draining with Pleurx®, intrapleural rituximab (100mg in 50ml of physiological saline solution every 3 weeks) was administered on cycle day +1 via this tube, clamping it for 2h.
The progress of the effusion was excellent, with persistence of only minimal blunting of the costophrenic angle; PF drainage did not need to be repeated in the next 2 cycles.
The patient died 1 month after the last intrapleural cycle (3 months after diagnosis) due to intracranial hemorrhage caused by neurological infiltration of the lymphoma.
Our second case was another 65-year-old woman with a 6-year history of IgA kappa multiple myeloma (MM). She progressed to symptomatic MM, and began second-line treatment with bortezomib–doxorubicin–dexamethasone (PAD), achieving partial response. The myeloma progressed with thoracic plasmacytomas, and a third line was started with lenalidomide–dexamethasone, resulting in complete response after 5 cycles.
In the seventh cycle, the patient came to the emergency department with respiratory failure and massive left PE. Analysis of the PF revealed pleural infiltration by MM. A diagnosis of pleural extramedullary progression was made, thoracocentesis was performed, and the effusion was drained via a chest tube.
A VRD regimen (bortezomib, lenalidomide and dexamethasone 2) was started, with administration of 2/3 of the planned bortezomib dose via Pleurx®, which was clamped for 2h and subsequently opened. Marked clinical improvement was achieved in the first cycle, with decreased fluid formation.
After the second cycle, no plasma cells were observed in PF and at the beginning of the third cycle, no effusion was observed on either X-ray or CT, so the tube was removed. No recurrence of PE was observed after 12 months of follow-up.
The PTC is a tunneled fenestrated silicone tube, secured subcutaneously with a profibrotic cuff. It features a one-way valve for PF drainage, preventing the entry of air or bacteria into the cavity. Its main indication is trapped lung, and it is the second choice in patients with MPE and a life expectancy of less than 3 months.1
Complications of chemical pleurodesis are potentially serious, including pneumonia, arrhythmia, respiratory failure, and respiratory distress, and mortality ranges between 6.1% and 8.4%, depending on the technique used.2
The main advantages of this type of catheter compared to conventional pleurodesis are improvement of dyspnea, shorter length of hospital stay, and lower costs. This is a valid, cost-effective alternative to pleurodesis in the treatment of this type of effusion.3
Complications include pleural cavity infection, metastasis along the trajectory of the tube, asymptomatic loculations, hypoproteinemia, breakage or dislodging of the tube, and chest pain.
Pleural infection is the complication that causes most concern. The incidence of this complication in individuals with MPE is estimated to range between 4.8% and 7%.4,5
The one-way valve is perforated with a specially equipped system, connected to a vacuum bottle or with a line connected to a suction system. The tube can be used either for draining fluid or for instilling drugs. These drugs can be either products that promote pleurodesis or chemotherapy drugs used locally.
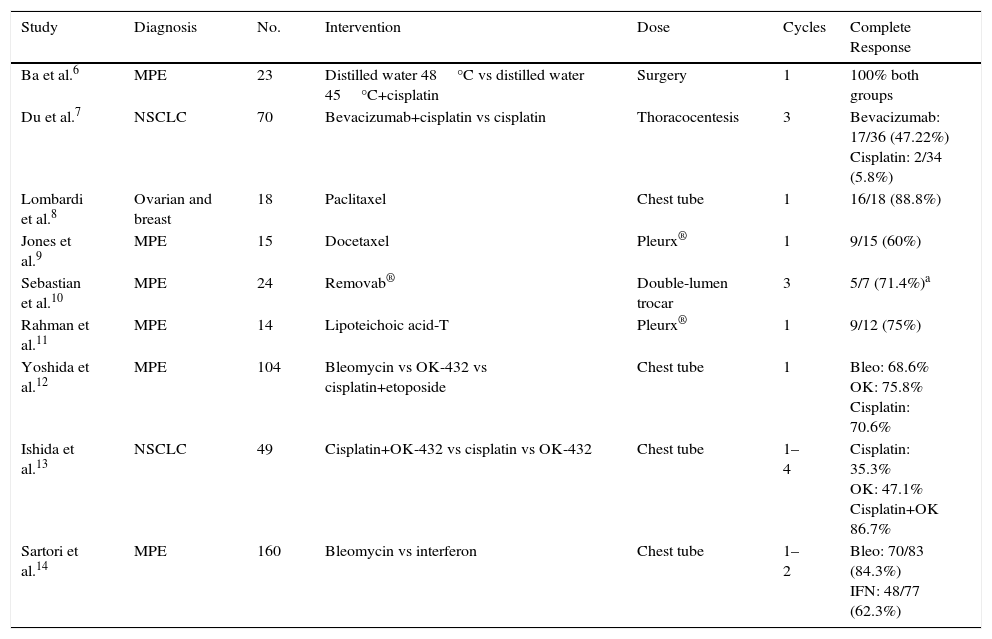
We performed a search of the literature for clinical trials (CT) in which the intrapleural instillation of chemotherapy drugs was studied. Trials published since 2004 were included, and data were collected on the instillation technique, the number of cycles, and results in terms of complete response and overall response at 1, 2, and 3 months. We found a total of 16 articles, of which 7 were excluded: 4 because the comparative technique was surgery, and the other 3 because their main endpoint was short-term survival, and no data were provided on the progress of pleural effusion. Some of the results are shown in Table 1.
Clinical Trials With Intrapleural Chemotherapy Administration, Route of Administration, and Outcome.
| Study | Diagnosis | No. | Intervention | Dose | Cycles | Complete Response |
|---|---|---|---|---|---|---|
| Ba et al.6 | MPE | 23 | Distilled water 48°C vs distilled water 45°C+cisplatin | Surgery | 1 | 100% both groups |
| Du et al.7 | NSCLC | 70 | Bevacizumab+cisplatin vs cisplatin | Thoracocentesis | 3 | Bevacizumab: 17/36 (47.22%) Cisplatin: 2/34 (5.8%) |
| Lombardi et al.8 | Ovarian and breast | 18 | Paclitaxel | Chest tube | 1 | 16/18 (88.8%) |
| Jones et al.9 | MPE | 15 | Docetaxel | Pleurx® | 1 | 9/15 (60%) |
| Sebastian et al.10 | MPE | 24 | Removab® | Double-lumen trocar | 3 | 5/7 (71.4%)a |
| Rahman et al.11 | MPE | 14 | Lipoteichoic acid-T | Pleurx® | 1 | 9/12 (75%) |
| Yoshida et al.12 | MPE | 104 | Bleomycin vs OK-432 vs cisplatin+etoposide | Chest tube | 1 | Bleo: 68.6% OK: 75.8% Cisplatin: 70.6% |
| Ishida et al.13 | NSCLC | 49 | Cisplatin+OK-432 vs cisplatin vs OK-432 | Chest tube | 1–4 | Cisplatin: 35.3% OK: 47.1% Cisplatin+OK 86.7% |
| Sartori et al.14 | MPE | 160 | Bleomycin vs interferon | Chest tube | 1–2 | Bleo: 70/83 (84.3%) IFN: 48/77 (62.3%) |
MPE: malignant pleural effusion; NSCLC: non-small cell lung cancer.
Our conclusion from this review is that the greater the number of cycles of intrapleural chemotherapy administered, the greater the chance of success. Higher rates of success were obtained in trials that used Pleurx®. We did not identify any CT that included data on hematological cancers, but case studies have been published on blood cancers with pleural infiltration treated with intrapleural instillation of chemotherapy.
Concomitant administration of systemic and local chemotherapy is a technique that has been used with satisfactory results in other organs, such as bladder tumors. This practice allows much higher drug concentrations to be achieved than by the systemic route, without the corresponding toxic effects. The limitations of this method lie in its limited capacity for dissemination throughout the pleura and poor penetration in bulky tumors.15
In conclusion, the administration of intrapleural chemotherapy may be an effective alternative for the treatment of intrapleural cancers. PTC is an effective, integrated solution to the problem of MPE. It provides fast relief of symptoms with improved quality of life; systemic chemotherapy can be used concomitantly; it is cost-effective; and the risk of complications is low. Moreover, it presents a new therapeutic target for the treatment of malignant infiltration of the pleura.
We thank Paloma Claveria Marco for her invaluable assistance.
Please cite this article as: Lázaro Sierra J, Carrasco V, Cases E, Gómez Gonzalez C. Utilización del catéter tunelizado permanente para administración de quimioterapia intrapleural a largo plazo: a propósito de 2 casos. Arch Bronconeumol. 2017;53:590–591.