Tuberculosis (TB) is one of the most common causes worldwide of morbidity and mortality due to infection, with a recorded incidence in Spain in 2015 of 21.5 cases/100000 inhabitants.1
Vasculitis is a general term for a heterogeneous group of diseases characterized by inflammation and destruction of blood vessel walls.2 Most cases are primary, but vasculitis can also be secondary to other diseases, including infections.3 At times the difference between TB and vasculitis can be difficult to determine, because they share similar characteristics, and moreover, both entities can coexist in the same patient.4 The definitions of vasculitis and its different forms are well established.5 We report the case of a patient that who presented simultaneous TB and microscopic polyangitis (MPA).
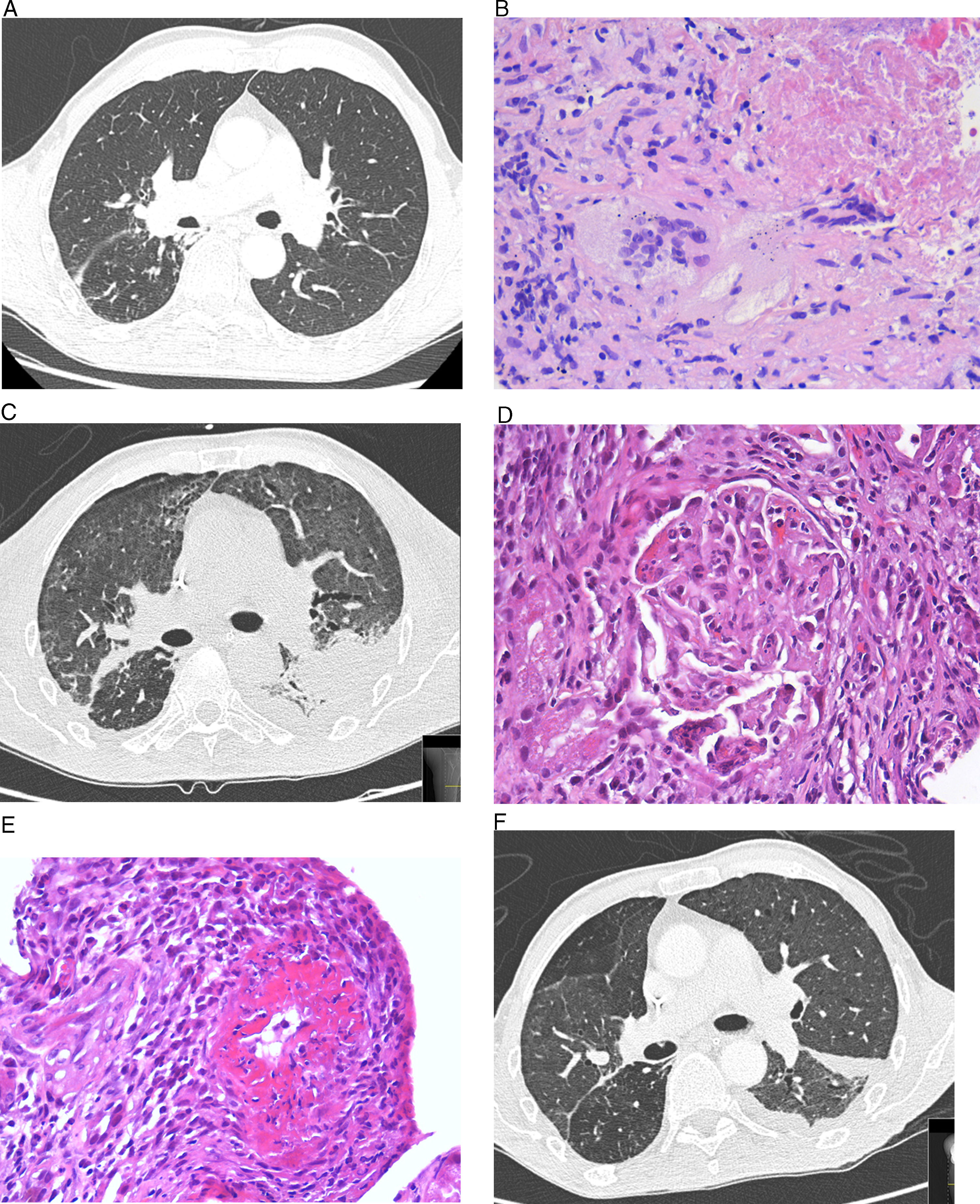
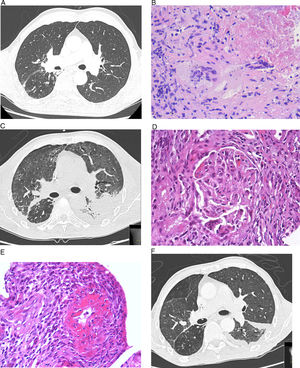
This was a 68-year-old man with a history of TB, who attended the emergency room with a 1-month history of fever, bloody sputum, asthenia, weight loss, and dyspnea. His temperature was 38.4°C, with no other significant findings. Blood tests were normal, and chest X-ray revealed scarring in the upper right lobe and a solid, spiculated parenchymal lesion. Chest computed tomography (CT) showed no changes in the middle and lower fields (Fig. 1A). No upper respiratory tract involvement was observed. Polymerase chain reaction was positive for Mycobacterium tuberculosis in bronchial aspirate and bronchoalveolar lavage. Core needle biopsy of the spiculated lesion revealed necrotizing granulomatous inflammation with multinucleated Langhans giant cells (Fig. 1B), and positive Ziehl–Neelsen staining and polymerase chain reaction for M. tuberculosis. The patient developed sudden onset hemoptysis with anemia (hemoglobin>6.8g/dL, hematocrit 20.8%), acute renal failure (urea 123mg/dL, creatinine 8.3mg/dL), oligoanuria and elevated transaminases (values 5 times the upper limit of normal). Intubation, mechanical ventilation, and hemodialysis were required. In view of the patient's hepatic and renal insufficiency, antituberculosis treatment began with ethambutol, levofloxacin, and streptomycin. Chest CT showed diffusely increased pulmonary radiodensity, mainly ground glass opacities and areas of consolidation in the peribroncovascular region, with moderate left loculated pleural effusion, with a fissural component, that was interpreted as diffuse alveolar hemorrhage (Fig. 1C). Renal biopsy revealed vasculitis with fibrinoid necrosis of the small arteries associated with focal and segmental necrotizing glomerulonephritis with an absence of immunoglobulin, complement and light chain deposits, suggestive of MPA (Fig. 1D and E). Pleural fluid was a lymphocytic exudate; ADA 45U/L, with no other significant changes. Anti-neutrophil cytoplasmic antibodies (ANCA) (dilution 1/320; p-ANCA pattern) with anti-myeloperoxidase antibodies>300IU/mL were detected. Anti-glomerular basement membrane antibodies were negative. Treatment was administered with corticosteroids (3 initial boluses of methylprednisolone 500mg/day, tapered to 15mg/day of prednisone), plasmapheresis (7 sessions), and rituximab (700mg/week for 4 weeks). Rifampicin and isoniazid could subsequently be reintroduced. Progress was slow but favorable, with stabilization of respiratory symptoms and radiological improvement (Fig. 1F).
(A) Chest high-resolution computed tomography (HRCT). No evidence of disease or pleural effusion observed in middle and lower fields. (B) Lung biopsy. Granulomatous inflammation with extensive areas of necrosis and multinucleated Langhans giant cells. (C) Chest HRCT. Diffuse increase of pulmonary radiodensity, mainly ground glass opacities with moderate left loculated pleural effusion, and a fissural component, interpreted as diffuse alveolar hemorrhage. (D) Renal biopsy. Renal glomerulus with area of focal necrosis and karyorrhexis. (E) Renal biopsy with fibrinoid necrosis of the small arteries affecting more than 50% of the circumference, associated with transmural inflammation. (F) Chest HRCT. Reduction of pleural effusion and slight improvement of the ground glass opacities.
The association between TB and vasculitis has been described, but generally always in association with granulomatosis with polyangitis.4,6,7 As far as we know, this is the second case in which TB has been associated with MPA.7 Both diagnoses appear to be confirmed: positive polymerase chain reaction in 2 different samples in the case of TB; and for MPA, granulomatous inflammation with necrosis and multinucleated giant cells in lung tissue with positive Ziehl–Neelsen staining, and confirmed diagnostic criteria with rapidly progressing diffuse alveolar hemorrhage and necrotizing glomerulonephritis.5
The question here is, is this association incidental or does one disease (TB) lead to another (MPA)? The inverse association is possible, and the administration of an immunosuppressive treatment for a diagnosis of vasculitis as a trigger for TB has been documented,8 but this could be ruled out in our patient by the order of events. Flores-Suárez et al. observed high odds ratios for positive p-ANCA by indirect immunofluorescence in TB patients, compared with asthma patients (odds ratio 2.96 [1.19–7.38]; P<0.027) and healthy subjects (odds ratio 18 [3.88–83.4]; P<0.0001).9 It is difficult to establish an association between TB and MPA, although the coexistence of TB and positive ANCA may be a causative factor. Some drugs, especially isoniazid and rifampicin, can be transformed into active metabolites that develop cytotoxic products that destroy the neutrophils with subsequent synthesis of ANCA, a phenomenon which might explain the presence of anti-myeloperoxidase antibodies in patients receiving these drugs.10,11 However, this is unlikely in our patient, because he had not yet received these products when the diffuse alveolar hemorrhage and necrotizing glomerulonephritis occurred. On the other hand, M. tuberculosis can stimulate the release of oxygen metabolites from the neutrophils. When these cells are activated in the initial stages of mycobacterial infection, lysosomal enzymes are released that could lead to the development of autoantibodies (ANCA) against the granular components of these cells.9 These IgG antibodies that act against neutrophilic and monocytic cytoplasmic antigens (proteinase-3 and myeloperoxidase) induce neutrophil migration and degranulation in the vessel wall, and release proteases and other toxic metabolites that cause vascular damage,12 which could give rise to this or any other vasculitis.
In summary, TB is more common in our setting than vasculitis, so diagnosis must be established promptly and treatment must be initiated in case of objective evidence. The characteristics of vasculitis and TB can overlap, and vasculitis should be considered in the differential diagnosis, particularly if azotemia is observed. Sometimes the possibility of a simultaneous presentation must be considered, and while no association between the 2 entities has been demonstrated, the mechanisms we describe may provide a physiopathological explanation. A high index of suspicion and clinical experience in the management of this presentation is necessary, since diagnostic errors and delays in treatments can lead to life-threatening situations.
Please cite this article as: Riveiro V, Ricoy J, Toubes ME, Valdés L. Tuberculosis y poliangitis microscópica. Una asociación muy poco frecuentex. Arch Bronconeumol. 2018;54:635–637.