To analyze the direct and indirect costs of diagnosis and management of tuberculosis (TB) and associated factors.
Patients and methodsProspective study of patients diagnosed with TB between September 2014 and September 2015. We calculated direct (hospital stays, visits, diagnostic tests, and treatment) and indirect (sick leave and loss of productivity, contact tracing, and rehabilitation) costs. The following cost-related variables were compared: age, gender, country of origin, hospital stays, diagnostic testing, sensitivity testing, treatment, resistance, directed observed therapy (DOT), and days of sick leave. Proportions were compared using the chi-squared test and significant variables were included in a logistic regression analysis to calculate odds ratio (OR) and corresponding 95% confidence intervals.
Results319 patients were included with a mean age of 56.72±20.79 years. The average cost was €10,262.62±14,961.66, which increased significantly when associated with hospital admission, polymerase chain reaction, sputum smears and cultures, sensitivity testing, chest computed tomography, pleural biopsy, drug treatment longer than nine months, DOT and sick leave. In the multivariate analysis, hospitalization (OR=96.8; CI 29–472), sensitivity testing (OR=4.34; CI 1.71–12.1), chest CT (OR=2.25; CI 1.08–4.77), DOT (OR=20.76; CI 4.11–148) and sick leave (OR=26.9; CI 8.51–122) showed an independent association with cost.
ConclusionTuberculosis gives rise to significant health spending. In order to reduce these costs, more control of transmission, and fewer hospital admissions would be required.
Analizar los costes directos e indirectos derivados del diagnóstico y tratamiento de la tuberculosis (TB) y sus factores asociados.
Pacientes y métodosEstudio prospectivo de pacientes diagnosticados de TB entre septiembre de 2014 y septiembre de 2015. Se calcularon los costes directos (estancias hospitalarias, consultas, estudios diagnósticos y tratamiento), e indirectos (absentismo laboral y pérdida de productividad, estudio de contactos y medidas rehabilitadoras). Los costes se compararon atendiendo a las variables: edad, sexo, país de origen, ingreso hospitalario, pruebas diagnósticas, tratamiento, resistencia farmacológica, tratamiento directamente observado (TDO) y días de baja laboral. Se compararon proporciones mediante Chi cuadrado y las variables significativas se incluyeron en un modelo de regresión logística calculándose las odds ratio (OR) y sus correspondientes intervalos de confianza del 95% (IC).
ResultadosFueron incluidos 319 pacientes con una edad media de 56,72±20,79 años. El coste medio fue de 10.262,62±14.961,66€, y aumentaba significativamente en relación con el ingreso hospitalario, el uso de la PCR, la realización de baciloscopia y cultivo, antibiograma, tomografía axial computarizada de tórax, biopsia pleural, tratamiento de más de 9 meses, TDO y baja laboral. En el análisis multivariante mantenían asociación independiente: ingreso hospitalario (OR=96,8; IC: 29-472,3), antibiograma (OR=4,34; IC: 1,71-12,1), tomografía axial computarizada de tórax (OR=2,25; IC: 1,08-4,77), TDO (OR=20,76; IC: 4,11-148) y baja laboral (OR=26,9; IC: 8,51-122).
ConclusiónLa Tuberculosis acarrea un gasto sanitario significativo. Medidas dirigidas a mejorar el control de la enfermedad y disminuir los ingresos hospitalarios serían importantes para reducirlo.
Tuberculosis (TB) continues to be the most common global disease, causing 1.5 million deaths in 2014, with approximately 9.6 million new cases occurring every year.1 In Spain, according to data from the Epidemiological Surveillance Network,2 5018 cases of TB were notified via the individualized mandatory reporting system in 2014, 3933 of which were classified as respiratory tuberculosis, with an incidence rate of 10.80 cases per 100,000 inhabitants. However, a study recently conducted by the Integrated Tuberculosis Research Program (PII TB) of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR)3 revealed significant under-reporting, ranging from 0% to 40% depending on the hospital, with a mean under-reporting rate of 14.4%.
The TB patient population worldwide and in Spain generates economic and social problems, giving rise to direct costs in the form of healthcare spending, and indirect costs, primarily in the form of days of work lost, and even years of life lost.4 Moreover, TB affects the working population and individuals with all kinds of difficulties (immigrants, HIV-infected individuals), so its socioeconomic impact is significant. Few studies have been published in Spain on the costs of TB,5–8 and those that have appeared are limited by their exclusive focus on hospitalization costs.
Our aim, then, was to analyze costs derived from the diagnosis and follow-up of TB and associated factors.
Patients and MethodsDesignProspective observational multicenter study.
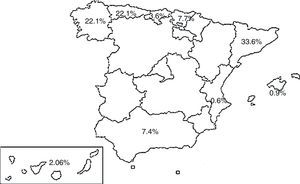
PopulationPatients with a diagnosis of TB registered in the SEPAR PII TB database between September 2014 and September 2015. Nineteen hospitals participated in 9 autonomous communities, and the cases were distributed according to the percentages shown in Fig. 1.
Informed consent was obtained from all subjects before inclusion, and the study was approved by the Regional Clinical Research Ethics Committee of the Principality of Asturias.
Inclusion CriteriaPatients were at least 18 years of age with a diagnosis of TB according to the following criteria:
- (1)
Positive or negative sputum smear with Mycobacterium tuberculosis isolated from culture medium.
- (2)
Caseifying granulomas detected in cases of extrapulmonary TB.
- (3)
Patients with clinical, radiological, epidemiological and/or laboratory suspicion justifying, in the opinion of the physician, anti-tuberculosis treatment.
All patients attended at least 3 visits: at the time of inclusion and at month 2 and 6 after starting treatment, during which laboratory, microbiological and radiological tests were performed and results recorded. Patients who needed to prolong therapy beyond 6 months, for whatever reason, were scheduled to return for another visit at month 9, and subsequently as judged appropriate until completion of treatment. Some patients attended more than 3 visits in the first 6 months for various reasons, including adverse reactions, delay in bacteriological conversion, suspected poor adherence, etc.
VariablesIndependent VariablesSociodemographic and anthropometric data were collected as well as smoking habit, concomitant diseases, diagnostic methods, treatment, drug susceptibility studies, treatment adherence, adverse drug reactions, side effects, need for hospital admission or sick leave.
Dependent VariablesDirect costs (hospitalization, visits, diagnostic tests, treatment) and indirect costs (derived from work absenteeism and loss of productivity, rehabilitation, and contact tracing) were estimated.
Cost Study. MeasuresFor the estimation of direct costs, we considered the cost of specialist health care derived from hospitalization (by day), outpatient visits (first and successive), imaging studies (standard radiography, chest computed tomography [CT]), laboratory testing (complete blood count, kidney and liver function tests), microbiology (sputum smear, mycobacterial culture, polymerase chain reaction [PCR], drug susceptibility testing), and other diagnostic methods (bronchoscopy, pleural, bronchial, percutaneous, transbronchial or extrathoracic biopsy). Tariffs were based on the official prices for public healthcare services published by the Health Services of the Principality of Asturias9 and the Canary Islands.10 The cost of drug treatment (standard or prolonged) was calculated on the basis of the maximum dose of drugs administered in a single daily dose, and the use or non-use of directly observed therapy (DOT) was also coded. Direct costs are shown in Table 1.
Direct Costs.
| Variable | Cost (€) |
|---|---|
| Hospital stay/day | 403.60 |
| Stay in other healthcare facility/day | 126.90 |
| Visit | |
| First | 120.30 |
| Successive | 72.20 |
| Laboratory tests (complete blood count, liver function) | 23.48 |
| Mycobacteria (sputum smear and culture) | 19.66 |
| Drug susceptibility testing | 9 |
| PCR | 93.65 |
| Standard chest radiograph | 9.51 |
| Chest CT with contrast medium | 202.20 |
| Bronchoscopy and bronchial aspiration | 151.66 |
| Bronchoscopy with biopsy | 215.46 |
| Pleural biopsy | 132 |
| CT-guided percutaneous biopsy | 266 |
| Extrathoracic biopsy | 63.80 |
| Treatment | |
| 6 months | |
| 3 drugs | 224.32 |
| 4 drugs | 206.52 |
| 9 months | 272 |
| Isoniazid resistance | 517.82 |
| Rifampicin resistance | 2079.81 |
| MDR | 7834.11 |
| DOT | 30/day |
MDR: resistance to rifampicin and isoniazid; CT: computed tomography; DOT: directly observed treatment; PCR: polymerase chain reaction.
All costs were compared in terms of the following variables: age, sex, origin (Spanish-born, immigrant), microbiological, imaging and histological studies, drug resistance, treatment, DOT, and need for admission to a hospital or other healthcare facility, in order to determine which variables impacted significantly on costs.
Indirect costs derived from work absenteeism and loss of productivity were estimated using the following formula: number of days of sick leave×cost per day of leave×proportion of population in active employment.11 The assumed cost per day was €95.8211 and the employment rate in Spain, retrieved from the official employment survey published by the National Institute of Statistics, was taken to be 0.59.12
The cost of rehabilitation measures (understood as treatments other than anti-tuberculosis therapy required during the course of the disease and after resolution) was included. These costs were considered to be incurred by 2.5% of patients for a mean duration of 24 days and a cost of €123, with a per-patient cost of €2952. Rehabilitation costs, then, were calculated as follows: €2952×0.025=€73.8 per case.11
Weighted costs incurred by contact tracing of the index case were also analyzed. Based on previous statistical studies, contact tracing was estimated to cost the healthcare system €921.80 per patient.13
Information gathered from each patient was recorded in an internet-based electronic case report form accessed via a website by each of the investigators participating in the study using a personalized user name and password. Completion of the surveys and the database were monitored by telephone and electronic mail.
Statistical AnalysisThe frequency distribution of variables of interest was analyzed and proportions between the groups were compared on a bivariate level using chi-squared distribution methods. In the bivariate study, the relationship between each of the variables and median costs (categorized as binary response variable) was analyzed. Variables which showed significance levels of P<.05 were included in a stepwise logistical regression model. Odds ratios (OR) and corresponding 95% confidence intervals (CI) were calculated. For all analyses, significance was set at P<.05 and statistical analysis was performed using IBM SPSS Statistics 19 software.
ResultsGeneral CharacteristicsCost data were available from 319 patients included in the study: 206 men and 113 women, with a mean age of 56.72±20.79 years. One hundred and three (32.3%) were immigrants and 74.4% presented pulmonary TB. Microbiological resistance was confirmed in 14 patients (4.4%): 10 to isoniazid, 2 to rifampicin, and 2 to isoniazid and rifampicin (MDR). During the diagnostic process, sputum smears were performed in 285 subjects (89.3%) (56% positive), PCR was determined in 201 (63%), CT in 141 (44.1%), bronchoscopy in 84 (26.3%) and biopsy in 95 (30.5%): 19 pleural, 18 transbronchial, 17 bronchial, 10 percutaneous, and 31 in other sites. Treatment regimens of 6 months were administered, with 4 drugs in 70.5% of cases and with 3 drugs in 20.7%. Cure or completion of treatment was achieved in 89.1%. DOT was performed in 67 patients (21%). A total of 187 patients (58.6%) were admitted to hospital for a mean duration of 11.3±7.09 days and 44 (13.8%) to other healthcare facilities for a mean duration of 31±90.65 days. The mean number of outpatient visits was 6.81±3.62. In total, 133 patients (41.7%) were in active employment and 89 (27.9%) needed to take sick leave lasting on average 21.74±55 days at some time during the course of treatment.
Cost StudyTotal cost was €3,188,477.39: €2,763,954.10 for direct costs and €424,523.29 for indirect costs (€106,926.89 due to loss of productivity, €294,054.20 for contact studies, and €23,542.20 for rehabilitation), with a mean cost per patient of €10,262.62±€14,961.66. Direct costs were distributed as follows: 61.4% derived from admission to hospitals and other healthcare facilities, 17.6% from treatment (including DOT), 13.4% from diagnostic studies (microbiological and histological), 5.2% from outpatient visits and laboratory tests, and 1.8% from imaging techniques.
As shown in Table 2, the bivariate analysis revealed that costs were associated with male sex, admission to a hospital or other healthcare facility, PCR, antibiotic resistance testing, chest CT, pleural biopsy, drug treatment lasting more than 9 months, DOT, and sick leave. The multivariate study showed an independent association between costs and the following variables: hospitalization (OR=96.8: CI: 29–472.3), drug susceptibility testing (OR=4.34; CI: 1.71–12.1), chest CT (OR=2.25; CI: 1.08–4.77), DOT (OR=20.76; CI: 4.11–148) and sick leave (OR=26.9; CI: 8.51–122).
Bivariate and Multivariate Analysis.
| Median Cost | Bivariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| <€4573 | ≥€4573 | OR | P-value | OR | P-value | |
| N (%) | N (%) | |||||
| Sex | ||||||
| Women | 63 (60.6) | 41 (39.4) | Ref. | Ref. | Ref. | Ref. |
| Men | 85 (43.1) | 112 (56.9) | 2.02 (1.25–3.29) | .004 | 2.20 (0.99–5.02) | .05 |
| Age | ||||||
| 18–30 | 39 (50.6) | 38 (49.4) | Ref. | Ref. | ||
| 31–50 | 68 (50) | 68 (50) | 1.03 (0.58–1.80) | .92 | ||
| >50 | 49 (48.5) | 52 (51.5) | 1.09 (0.60–1.98) | .78 | ||
| Country of origin | ||||||
| Spanish-born | 115 (53.2) | 101 (46.8) | Ref. | Ref. | ||
| Immigrant | 44 (42.7) | 59 (57.3) | 1.52 (0.95–2.46) | .08 | ||
| Hospital admission | ||||||
| No | 111 (84.1) | 21 (15.9) | Ref. | Ref. | Ref. | Ref. |
| Yes | 48 (25.7) | 139 (74.3) | 15.1 (8.66–27.3) | <.001 | 96.8 (29–472.3) | <.001 |
| Other healthcare facility | ||||||
| No | 158 (57.5) | 117 (42.5) | Ref. | Ref. | Ref. | Ref. |
| Yes | 1 (2.3) | 43 (97.7) | 50.6 (10.9–119.3) | <.001 | 4.29 (0.67–8.45) | .19 |
| PCR | ||||||
| No | 77 (65.3) | 41 (34.7) | Ref. | Ref. | Ref. | Ref. |
| Yes | 82 (40.8) | 119 (50.2) | 2.71 (1.70–4.38) | <.001 | 2.06 (1.09–11.93) | .06 |
| SS and culture | ||||||
| No | 25 (73.5) | 9 (26.5) | Ref. | Ref. | ||
| Yes | 134 (47) | 151 (53) | 3.09 (1.43–7.27) | .004 | ||
| Drug susceptibilty testing | ||||||
| No | 59 (70.2) | 25 (29.8) | Ref. | Ref. | Ref. | Ref. |
| Yes | 100 (42.6) | 135 (57.4) | 3.16 (1.87–5.48) | <.001 | 4.34 (1.71–12.1) | .003 |
| Resistance | ||||||
| No | 153 (50.2) | 152 (49.8) | Ref. | Ref. | ||
| Yes | 6 (42.9) | 8 (57.1) | 1.13 (0.44–4.23) | .6 | ||
| Chest CT | ||||||
| No | 104 (58.4) | 74 (41.6) | Ref. | Ref. | Ref. | Ref. |
| Yes | 55 (39) | 86 (61) | 2.19 (1.40–3.46) | .001 | 2.25 (1.08–4.77) | .03 |
| Pleural biopsy | ||||||
| No | 155 (51.7) | 145 (48.3) | Ref. | Ref. | Ref. | Ref. |
| Yes | 4 (21.1) | 15 (78.9) | 3.89 (1.36–14.3) | .01 | 4.70 (0.95–28.77) | .06 |
| Bronchoscopy | ||||||
| No | 46 (54.8) | 38 (45.2) | Ref. | Ref. | ||
| Yes | 113 (48.1) | 122 (51.9) | 1.31 (0.79–2.16) | .29 | ||
| Treatment | ||||||
| 6 months | 121 (53.8) | 104 (46.2) | Ref. | Ref. | Ref. | Ref. |
| 9 months | 31 (47) | 35 (53) | 1.31 (0.76–2.29) | .33 | ||
| Prolonged | 7 (25) | 21 (75) | 3.43 (1.45–9.11) | .004 | 2.26 (0.95–8.77) | .06 |
| DOT | ||||||
| No | 154 (61.1) | 98 (38.9) | Ref. | Ref. | Ref. | Ref. |
| Yes | 5 (7.5) | 62 (92.5) | 18.81 (7.79–56.1) | <.001 | 20.7 (4.11–148) | <.001 |
| Sick leave | ||||||
| No | 128 (55.7) | 102 (44.3) | Ref. | Ref. | Ref. | Ref. |
| Yes | 31 (34.8) | 58 (65.2) | 2.34 (1.41–3.92) | .001 | 26.9 (8.51–122) | <.001 |
SS and culture: sputum smear and mycobacterial culture; OR: odds ratio; PCR: polymerase chain reaction; Ref.: reference; CT: computed tomography.
Several original articles have been published in recent years on the costs generated by various aspects of tuberculosis, showing how the distribution of costs varies depending on the economic resources of the country in question. The fewer the resources, the greater the spending derived from indirect costs in the post-diagnosis period, while in more highly developed regions, direct costs, in particular those generated by hospitalization, have the most impact.14
A recent study of all series published in the European Union, of which only 1 was conducted in Spain, showed that mean per-patient cost of drug-sensitive tuberculosis was €10,282 and €57,213 for MDR, 77% of which was derived from direct costs.15 In our study, which had a similar design, the percentage of expenditure attributed to direct costs was 86%, and mean cost per patient was lower, perhaps due to the small percentage of cases with resistance, including MDR. This is in line with previous studies in Spain that reported an overall rate of 9.2% for drug resistance and 1.9% for MDR.16 The lower impact of indirect costs may derive from our use of an employment rate of 59%, retrieved from the official employment survey data, for the calculation of costs derived from loss of productivity, in contrast to the 71% employment rate reported in the aforementioned series.15 Basing our calculations on a rate of 71% would have increased indirect costs by approximately 16%, which would give us a result more in line with that obtained in the European study. Nevertheless, that study showed striking variations among our neighboring countries, with mean costs ranging from €5691 to €8242 per patient. These differences were supported by a study performed in Denmark where the mean direct cost of TB was €10,509.17 In this country, as in Spain, the resistance rate is low, and therefore the results of the Danish authors are similar to ours.
In our series, costs were determined by hospitalization (accounting for 61% of total expenditure), drug susceptibility testing, DOT, sick leave, and chest CT. The impact of hospital costs has already been addressed by Montes-Santiago et al.,8 who observed that in Spain in 2006, the cost of hospitalization for tuberculosis amounted to €30.8 million. Assuming that hospitalization accounts for 70% of costs, the total cost of tuberculosis was €40 million, representing 0.7% of total public expenditure. These authors also observed a slight fall in costs coinciding with fewer hospital admissions between 1999 and 2006. Similarly, in another series conducted in Germany,12 the cost of a hospital stay was practically 9 times that of treating the patient on an exclusively outpatient basis. The same occurred in Denmark, where hospitalization accounts for 81% of costs.17 Furthermore, in a national tuberculosis program in South Africa, Pooran et al.18 determined that costs generated by hospitalization accounted for a significant portion of total costs: 56% in their case. Considering the above, hospital admission for TB should be avoided as far as possible and limited to cases recommended in clinical guidelines19 (severe cases or disease complications and impossibility to isolate the patient at home), in order to reduce the risk of transmission and disease-related costs. Nonetheless, the percentage of hospital admissions for tuberculosis remains high, both in our sample, where it was 59%, and in other national and international reports, which range from 52% to 78%,7,8,11,20,21 although the reason for this high proportion remains unclear. Thus, a strategy aimed at reducing hospital stays would reduce costs, as has already been observed in some regions which have managed to achieve rates as low as 34%.7 If we take this figure and extrapolate our results to the number of TB cases notified in Spain in 2014, we estimate that savings of €14 million could be achieved.
Steffen et al.22 found that costs of DOT were significantly higher than the cost of self-administered treatment, but that it increased, albeit insignificantly, the rate of treatment completion. Furthermore, a recent meta-analysis of studies performed mainly in south-east Asia and English-speaking countries showed that DOT does not improve outcomes in terms of microbiological cure, prevention of relapse or development of acquired resistance compared to self-administered medication,23 so it seems reasonable to limit the use of DOT in countries with low to medium incidence to populations in special situations. In our setting, it is reserved solely for complicated cases or individuals with severe social problems who cannot follow their regimen normally. DOT is currently used in less than 25% of patients.
Our results also suggest that expenditure can also be reduced by decreasing the duration of sick leave, since earlier studies have shown that it has a significant impact on costs.17 We must bear in mind that the main objective is to reduce the transmission of the disease and that, with standard treatment, contagiousness is significantly reduced after 3 weeks.19 After that time, isolation measures can be lifted and the subject can return to the workplace. This period coincides with the mean number of days of sick leave identified in our study, so we can surmise that this aspect is not one of the most important in our setting.
From all of the above, then, we can conclude that avoiding unnecessary hospital stays is the only way to reduce costs. Nevertheless, it is important to point out that, since TB gives rise to significant healthcare costs (extrapolating the data from our study to cases notified in 2015, expenditure would amount to €51,500,000), reducing the number of cases is the surest way to reduce costs. This, in turn, highlights the importance of such proven cost-effective strategies as contact tracing and treatment of latent tuberculous infection.
Our study is limited in several ways. Similarly to the studies discussed above,12,13 we have only included estimated indirect costs derived from loss of productivity, rehabilitation therapies, and contact tracing, and have probably underestimated the real costs by failing to include other indirect costs, such as transport, food, electricity, maintenance, and human resources incurred by personnel caring for these patients, and the costs of primary care. However, many of these costs would have greater impact in countries lacking in resources and universal healthcare provision, or in populations with a significant proportion of patients with special requirements (which does not seem to be the case in our series). Moreover, the diagnosis and follow-up of these patients is generally performed in specialized care. We also did not include costs derived from pharmacological side effects, since most of them are reversible after discontinuation of the causative drug, and do not require further measures. The cost of adjuvant medication for gastrointestinal side effects is negligible. We therefore believe that, while it may be interesting to include these costs, they would not significantly affect the final outcomes of our study, and excluding them does not affect the validity of our conclusions. Finally, we used costing data from only 2 health services in our calculations, so we are aware that due to the variations between the different autonomous communities and the fact that only 9 of these participated in the study, our study may not reflect the exact cost of tuberculosis. However, we would like to point out that we aimed to use our data to obtain an approximate estimation, and we believe that we have achieved this in a reasonable enough manner for our study to have meaning in this setting.
ConclusionsTuberculosis gives rise to significant healthcare costs, generated by hospitalizations, imaging studies, directly observed treatment and the need for sick leave. Hospital admission appears to be the factor with the most impact, so strategies aimed at decreasing hospital stays would be essential if costs have to be reduced.
FundingThis project was funded by SEPAR grant 136/2013.
AuthorshipJosé Antonio Gullón: study concept. Writing, critical reading, review and final approval of the manuscript. Analysis of results.
José María García: study concept. Critical reading, review and final approval of the manuscript. Analysis of results.
Manuel Ángel Villanueva and Fernando Álvarez-Navascues: study concept. Analysis of results.
Teresa Rodrigo and Joan Caylá: critical reading, review and final approval of the manuscript. Analysis of results.
Martí Casals: statistical analysis.
Marta García-Clemente, Luis Anibarro, María Ángeles Jiménez, Ana Bustamante, Antón Penas and José Antonio Caminero: critical reading, review and final approval of the manuscript.
PII TB Working Group: data collection.
Conflict of InterestsThe authors state that they have no conflict of interests.
N. Altet (Serveis Clinics, Barcelona); E. Bikuña (Hospital de Zumárraga, Zumárraga); E. Carrió (Centro Asistencial Dr. Emili Mira i López); X. Casas (Hospital de Sant Boi, Llobregat); F.J. Garros (Hospital Santa Marina, Bilbao); M. Jiménez (Hospital Universitario de Donostia, Donostia); I. López (Hospital Universitario de Cruces, Barakaldo); M. Marín (Hospital General de Castellón, Castellón); J.F. Medina (Hospital Universitario Virgen del Rocío, Sevilla); J.P. Millet (Serveis Clinics, Barcelona); I. Mir (Hospital Son Llatzer, Palma de Mallorca); Laura Rodríguez (Hospital Universitario Germans Trías i Pujol, Badalona); F. Sánchez (Hospital del Mar, Barcelona).
Please cite this article as: Gullón JA, García-García JM, Villanueva MÁ, Álvarez-Navascues F, Rodrigo T, Casals M, et al. Costes de la tuberculosis en España: factores relacionados. Arch Bronconeumol. 2016;52:583–589.