Smoking is the leading cause of chronic obstructive pulmonary disease (COPD). Around 85%–90% of cases of this disease are due to tobacco smoking.1 It is estimated that between 15% and 20% of smokers may develop COPD over their lifetime. No one knows exactly why some smokers develop COPD and others do not, although it is believed that there may be a certain genetic predisposition in this regard.2
It is notable that a large number of COPD patients smoke, even when they know that they have this condition and that it is adversely affecting the course of their disease. Data from the IBERPOC study, and later those from another study conducted in England, found that between 30% and 70% of patients with COPD continue smoking, despite being diagnosed with the illness.3,4 Spanish Guidelines have recently been published for the diagnosis and treatment of patients with COPD, in which the different recommendations for the proper approach to these patients were considered.5 Furthermore, a recent study found that up to 20% of patients with COPD admitted to Spanish hospitals smoke.6
Several studies have shown that smokers with COPD have special characteristics in their smoking that make them a group with particular difficulties in quitting. The following have been described among these characteristics: (a) they smoke more cigarettes daily than smokers without COPD and moreover, they have a specific smoking pattern: they inhale the cigarette smoke deeply and retain it for longer in their lungs.4,7 In addition, the expired air carbon monoxide (CO) levels of these smokers are higher than in smokers without COPD4,6; (b) they have a higher degree of physical dependence on nicotine4,6; (c) they have a lower degree of motivation to quit smoking, low self-efficacy (this term defines the confidence that the subject has in himself to achieve smoking cessation) and their self-esteem levels are decreased4,6,8; (d) depression and depressed moods are common in this group of smokers9,10; and (e) the weight gain that is normally associated with smoking cessation can cause relapse in this patient group, as it may cause them to have more symptoms.
It is important to stress that the only measure that has been shown to be effective for arresting the progression of this disease is smoking cessation.11 This is why it is so important to stage a proper diagnostic and therapeutic intervention for smoking in these subjects.
Current Status of Treatment for Smoking in Patients With Chronic Obstructive Pulmonary DiseaseFew clinical trials have been conducted to study the efficacy and safety of the use of different pharmacological treatments for smoking in smokers with COPD. These are reviewed briefly below.
The Lung Health Study showed that using nicotine gum combined with intensive cognitive behavioural therapy (CBT) was effective and safe for helping these subjects to quit smoking.11,12 Later, Tønnesen and Mikkelsen,13 in a randomised, open-label study in which they used 4 forms of nicotine replacement therapy (NRT), found that, at the one-year follow-up, those who had received active treatment quit smoking more often than those who used placebo (5.6%; P<.01). These same authors, in another randomised, double-blind, placebo-controlled study, analysed the efficacy of sublingual nicotine tablets in 2 groups of patients: one that received intensive CBT and another that received mild intensity CBT. The abstinence rates at 6 and 12 months of follow-up were higher for the groups that received active treatment: 23% compared to 10% and 17% compared to 10%, respectively. No significant differences were observed according to the intensity of CBT received.14 More recently, a meta-analysis evaluated 7332 patients with COPD who received different smoking cessation treatments. The authors found that the combination of CBT plus NRT was the most effective type of intervention (OR: 5.08; P<.0001) compared to CBT alone (OR: 2.8; P=.001) and compared to CBT combined with an antidepressant (OR: 3.32; P=.002).15
Two clinical trials have analysed the efficacy and safety of the use of bupropion in the treatment of smokers with COPD. One of them found that bupropion was more effective than placebo in obtaining continuous abstinence at 6 months of follow-up (16% compared to 9%; P<.05).16 The other showed similar results, also at 6 months of follow-up, with a difference of 18.9% (95% CI: 3.6%–34.2%; P=.02).17
Two studies have analysed the efficacy and safety of the use of varenicline on the treatment of smoking in smokers with COPD. One was conducted on a group of patients with mild or moderate COPD and was designed as a randomised, double-blind, placebo-controlled study. It was shown that varenicline was more efficacious than placebo for aiding smoking cessation in the third, sixth and twelfth month of follow-up. After one year the continuous abstinence rates were 18.6% versus 5.6% (OR: 4.04 [95% CI: 2.13–7.67]; P<.0001).18 The other was an open-label, follow-up study in which a total of 472 smokers with severe or very severe COPD who received treatment with different drugs were studied: NRT, bupropion and varenicline. Patients were followed-up for 24 weeks. The mean continuous abstinence rate between week 9 and week 24 was 48.5%. Depending on the type of drug used, the rates were: 38.2% for NRT, 60% for bupropion and 61% for varenicline. Varenicline was more effective than nicotine patches, 61% versus 44.1% (OR: 1.98 [95% CI: 1.25–3.12]; P=.003).19
Another study compared the efficacy of a high intensity smoking treatment programme in smokers with COPD with those who received usual care. The high intensity programme consisted of a combination of pharmacological treatment plus hospitalisation for 2 weeks, where patients received intensive CBT; telephone calls and continuous follow-up were also scheduled for a period of one to 3 years. The abstinence rates in the group who received intensive treatment were 52% and 38% after one and 3 years of follow-up, respectively, while the rates in the group who received usual care were 7% and 10%, respectively.20
Probably one of the most informative studies was that by Hoogendoorn et al.,21 who carried out a systematic review of the different clinical trials on smokers with COPD. The various interventions were grouped into 4 categories: usual care, minimal intervention, intensive CBT and intensive CBT plus pharmacological treatment. The abstinence rates after one year of follow-up for each of the categories were 1.4%, 2.6%, 6.0%, and 12.3%, respectively. Compared with usual care, the cost per quality-adjusted life year for the minimal intervention was 16900Euros, for intensive CBT 8200Euros and for intensive CBT plus pharmacological treatment, 2400Euros. The authors concluded that the combination of intensive CBT plus pharmacological treatment is the most effective form of treatment for smoking in COPD, with the best cost/effectiveness ratio.21
Assessment of Smoking in Smokers With Chronic Obstructive Pulmonary DiseaseThis intervention should be performed differently depending on whether the patient has been recently diagnosed with COPD or if, on the contrary, the patient has been diagnosed with the disease for some time.
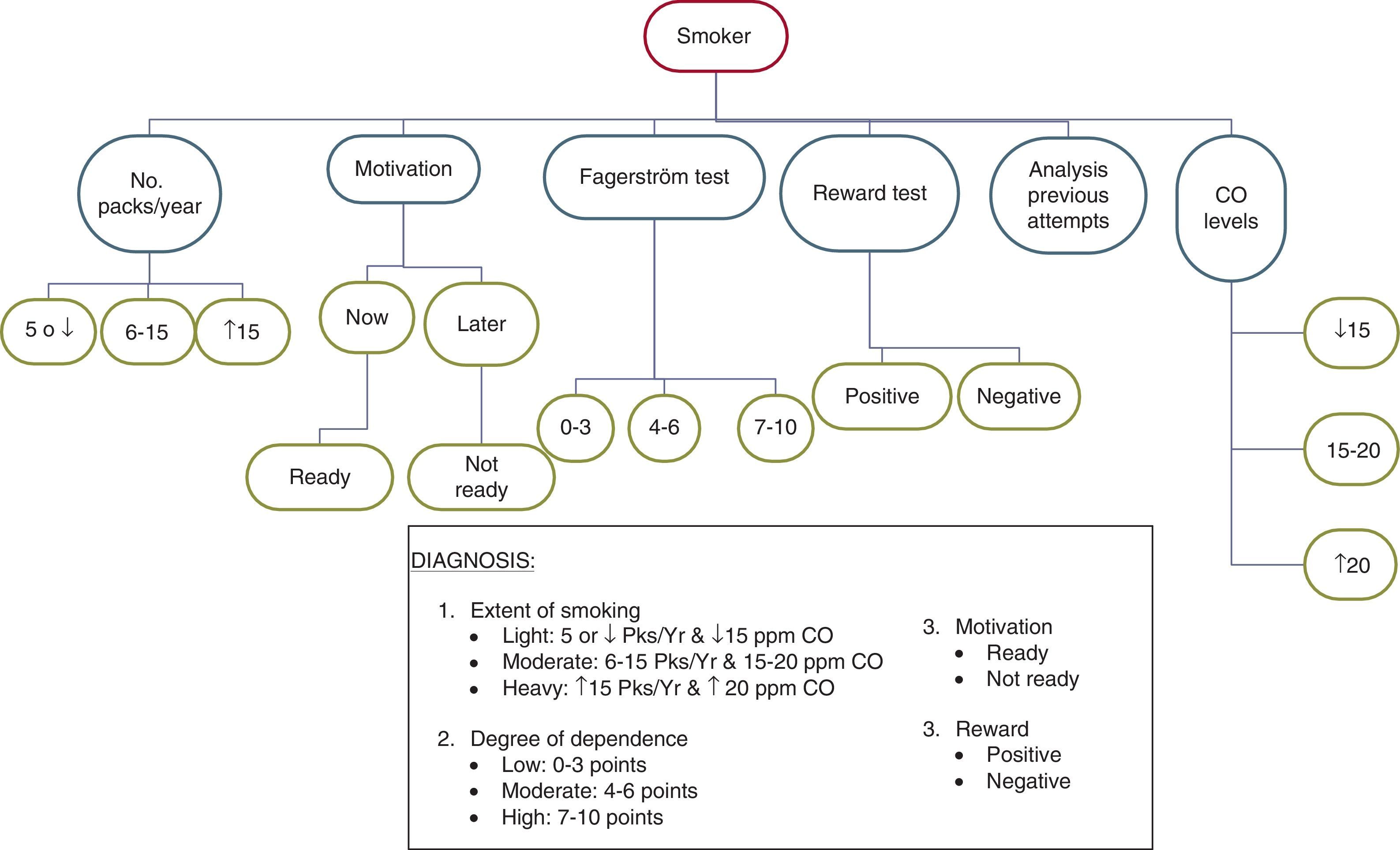
Diagnosis of Smoking in Smokers With Recently Diagnosed Chronic Obstructive Pulmonary DiseaseIn order to carry out this aspect, the following protocol should be followed: (a) determine the number of packs/year; (b) identify the degree of motivation to quit smoking; (c) study the degree of physical dependence on nicotine using the Fagerström test; (d) reward test; (e) analyse previous smoking cessation attempts; and (f) determine the expired air CO levels in the subject. Occasionally, and if available, the determination of cotinine levels in body fluids, especially serum, may be useful.22
- (a)
Determine the number of packs/year. Knowing this figure is helpful for assessing the prognosis. Patients with high numbers (more than 5packs/year) will have more difficulty in quitting smoking than those with lower numbers.
- (b)
Analyse the motivation to quit smoking. In this respect, subjects can be classified into 2 groups: those who are willing to make a serious attempt to quit at the present time, and those to prefer to delay the attempt until later.
- (c)
Study the degree of physical dependence on nicotine. The Fagerström test provides relevant information on this aspect. Table 1 shows this test.23 Of all the questions that comprise it, those that best define the degree of dependence are shown in Table 2 (Heaviness of Smoking Index).24
Table 1.Modified Fagerström Test.
• How soon after you wake up do you smoke your first cigarette? – Within 5min 3 – 6–30min 2 – 31–60min 1 – After 60min 0 • Do you find it difficult to refrain from smoking in places where it is forbidden (hospital, cinema, library)? – Yes 1 – No 0 • Which cigarette would you most hate to give up? – The first one in the morning 1 – All others 0 • How many cigarettes do you smoke a day? – Less than 10cigarettes/day 0 – Between 11 and 20cigarettes/day 1 – Between 21 and 30cigarettes/day 2 – 31 or more cigarettes/day 3 • Do you smoke more frequently during the first hours after waking than during the rest of the day? – Yes 1 – No 0 • Do you smoke even if you are so ill that you are in bed most of the day? – Yes 1 – No 0 Total Score: 0–3: low dependence 4–6: moderate dependence 7–10: high dependence Table 2.Heaviness of Smoking Index.
• How soon after you wake up do you smoke your first cigarette? – Within 5min 3 – 6–30min 2 – 31–60min 1 – After 60min 0 • How many cigarettes do you smoke a day? – Less than 10cigarettes/day 0 – Between 11 and 20cigarettes/day 1 – Between 21 and 30cigarettes/day 2 – 31 or more cigarettes/day 3 Score: 0–2: low dependence 3–4: moderate dependence 5–6: high dependence - (d)
Reward test. This test has also been shown to be quite useful for the assessment of smoking.25Table 3 shows this test and its scoring system.
Table 3.Reward Test.
When you try to quit smoking, which of these situations do you find most difficult? You most choose one of these two answers. Indicate the one which is most crucial for you. A. Continuously anxious, irritable, depressed. B. Not being able to smoke at times when you genuinely enjoy the pleasure of smoking a cigarette. Answer A Negative reward Answer B Positive reward - (e)
Analysis of previous attempts to quit smoking. This study is very helpful for determining the subject's smoking characteristics. It should be noted that for performing this analysis, only attempts that led to the subject not smoking for at least 24h should be considered. The following variables should be determined: number of attempts made, time without smoking at each attempt, symptoms suffered, treatments used and their effects, and finally, the reasons for relapse.
- (f)
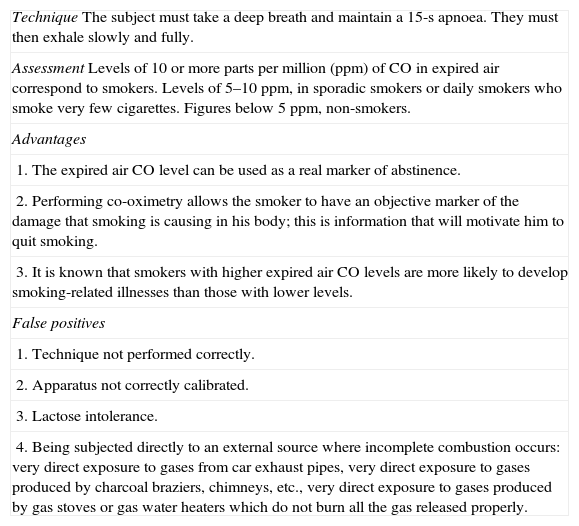
Determination of expired air CO levels. This is a simple examination. Table 4 shows how it should be performed and its assessment.26 This test not only should be used to confirm the patient's abstinence or to determine the amount of tobacco smoked, but it is also highly recommended for use as a motivational tool to quit smoking. After it has been performed, the healthcare professional should explain to the patient why he has high CO levels and how these high CO levels are causing the disease in his body. He should also be informed that once he has quit smoking, the CO levels will return to normal.
Table 4.Co-oximetry Technique, Assessment, Advantages and False Positives.
TechniqueThe subject must take a deep breath and maintain a 15-s apnoea. They must then exhale slowly and fully. AssessmentLevels of 10 or more parts per million (ppm) of CO in expired air correspond to smokers. Levels of 5–10ppm, in sporadic smokers or daily smokers who smoke very few cigarettes. Figures below 5ppm, non-smokers. Advantages 1. The expired air CO level can be used as a real marker of abstinence. 2. Performing co-oximetry allows the smoker to have an objective marker of the damage that smoking is causing in his body; this is information that will motivate him to quit smoking. 3. It is known that smokers with higher expired air CO levels are more likely to develop smoking-related illnesses than those with lower levels. False positives 1. Technique not performed correctly. 2. Apparatus not correctly calibrated. 3. Lactose intolerance. 4. Being subjected directly to an external source where incomplete combustion occurs: very direct exposure to gases from car exhaust pipes, very direct exposure to gases produced by charcoal braziers, chimneys, etc., very direct exposure to gases produced by gas stoves or gas water heaters which do not burn all the gas released properly.
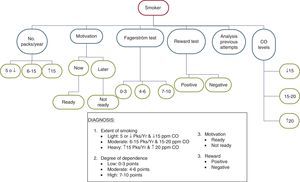
Taking into account all the data obtained on analysing the different variables, the diagnosis of smoking in the patient can be made according to 4 aspects: (a) extent of smoking; (b) motivation for smoking cessation; (c) degree of physical dependence on smoking; and (d) type of reward.
With respect to the extent of smoking, 3 types can be distinguished in turn: light (smokers of 5 or fewer packs/year and CO levels less than 15ppm), moderate (smokers of 6–15packs/year and CO levels between 15 and 20ppm) and heavy (smokers of more than 15packs/year and CO levels higher than 20ppm). The parameter “CO levels” is the most significant for establishing the classification.
With respect to the motivation, 2 patient groups can be distinguished: one group made up of subjects who are willing to make a serious attempt to quit at the present time (this group corresponds to smokers in the preparation stage of the Prochaszka classification) and another made up of subjects who do not want to make a serious attempt to quit smoking at the present time, and who prefer to delay the decision-making (this group corresponds to smokers in the pre-contemplation or contemplation phase of the Prochaszka classification).27,28
Depending on the degree of dependence, smokers can be classified into 3 types: light, moderate or heavy. Tables 1 and 2 explain each of these.
With respect to the type of reward, the keys are discussed in Table 3. Fig. 1 explains the diagnostic process in these subjects.
Diagnosis of Smoking in Smokers With Previously Diagnosed Chronic Obstructive Pulmonary DiseaseUp to 30%–70% of patients with COPD continue smoking, despite having been diagnosed with this condition and having been advised by their doctor on several occasions on the benefits of smoking cessation. In this patient group, the diagnosis of smoking has specific characteristics and should be made with empathy, respect and understanding by the doctor towards their patient.
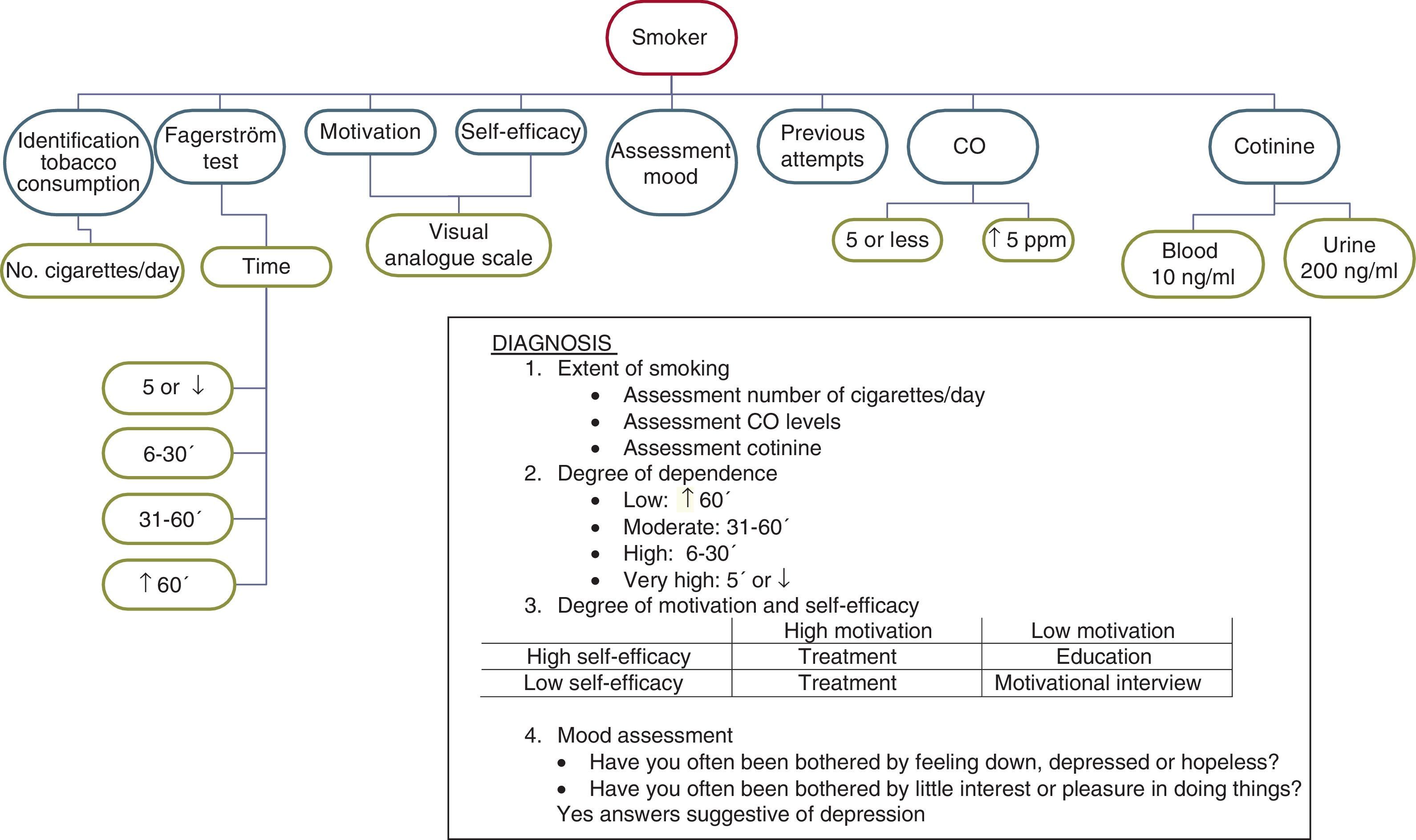
The most important aspects that must be addressed in this subject group are the following: identification of tobacco smoking; co-oximetry; determination of cotinine levels in body fluids, especially serum; analysis of degree of physical dependence on nicotine; analysis of degree of motivation to quit smoking; self-efficacy analysis; assessment of mood status, and analysis of previous attempts at smoking cessation.
The identification of tobacco smoking in these patients is a task that should be approached with empathy. Taking into account that on many occasions the subject may be reluctant to provide the real information, it is advisable to identify how many cigarettes he smokes per day and for how long he has smoked, despite knowing that it had been advised against. The use of co-oximetry and even the determination of cotinine levels are very useful for making, not only the tobacco consumption, but also the amount consumed objectively clear. In this respect, it is highly recommended that after having asked the subject about their smoking and the number of cigarettes smoked per day, the expired air CO levels are determined. Although CO levels less than 10ppm are traditionally considered to define non-smoking, various studies have found that the figure that marks the cut-off point is around 4–5ppm.26,29,30 Therefore, any subject who shows CO levels higher than 5ppm should be considered a smoker, providing that the examination has been correctly carried out and all cases of “false positive” co-oximetry have been ruled out (Table 4).
The determination of cotinine levels in serum, urine and saliva can also be very helpful for the reliable identification of smoking. The cut-off point for cotinine in plasma or saliva is 10ng/ml, and in urine is 200ng/ml.31
The Fagerström test and Heaviness of Smoking Index have been shown to be useful in these patients for diagnosing the degree of physical dependence on nicotine.23,24 Nevertheless, it is important to highlight that in this group of smokers, suffering from this chronic respiratory condition does not allow them to smoke a large number of cigarettes per day. Therefore, the answer that the subjects give to the question regarding the time between getting up until they smoke their first cigarette of the day is much more valuable than the answer that they give to the question regarding the number of cigarettes that they smoke daily.24
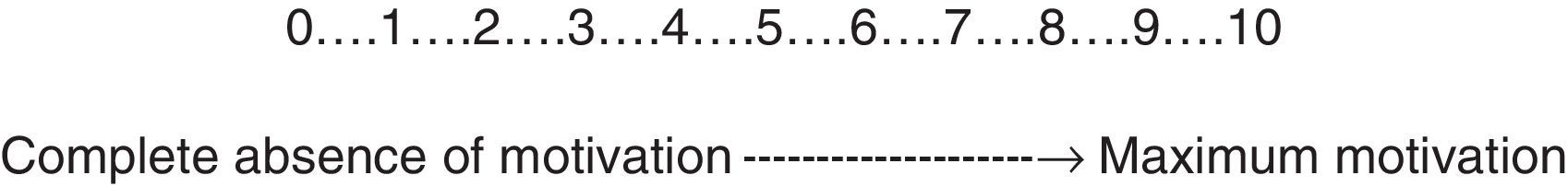
Analysis of the degree of motivation to quit smoking in these patients should be performed using a visual analogue scale (Fig. 2). It is advisable to proceed as follows: show the patient the figure and explain that this is a scale on which 0 corresponds to the total absence of motivation for quitting smoking and 10 to the maximum degree of motivation for doing so. Ask the patient to point with their finger to the point on the scale that best defines their degree of motivation, taking into account the rating explained above.32 The self-efficacy analysis and mood assessment are equally important in this patient group. Unfortunately, there are no scales that can accurately measure the self-efficacy of the subject for quitting smoking. It is recommendable to use the same visual analogue scale as used in measuring the motivation for this purpose, and to do so in a similar way to that used to determine the motivation.32
Assessing the mood is essential in these patients. Many studies have shown that depression is a very common comorbidity in subjects with COPD, and even those with more severe COPD suffer this condition more frequently.9,10 These reasons explain the need to diagnose the presence and degree of depression in these patients. Therefore, it is recommendable to ask the subject 2 questions: (a) during the past month, have you often been bothered by feeling down, depressed or hopeless? and (b) during the past month, have you often been bothered by little interest or pleasure in doing things? When the answer to both questions is yes, the likelihood that the subject has depression is very high.33
Analysis of previous attempts at smoking cessation in these subjects does not vary with respect to the analysis performed in the group of smokers with recently diagnosed COPD.
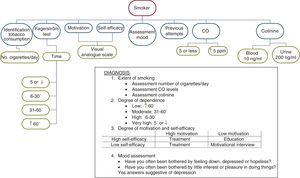
Taking into account the different results obtained after the assessment carried out in this subject group, we can diagnose patients according to the following criteria: (a) extent of smoking; (b) degree of physical dependence on nicotine; (c) degree of motivation and self-efficacy; and (d) assessment of mood.
- (a)
Assessment of the extent of smoking. For this, it is advisable to analyse the number of cigarettes smoked per day, the expired air CO levels and, if available, blood cotinine levels. In this subject group, there is little relation between the number of cigarettes smoked per day and the CO or blood cotinine levels. It is often observed that although they smoke a small number of cigarettes per day, the figures for expired air CO, or even blood cotinine levels, are higher than they should be. This is because these smokers smoke with a specific pattern: although they smoke fewer cigarettes, they take many puffs of the cigarette, inhale them deeply and retain the smoke inside their lungs for a long time.7,34
- (b)
Assessment of the degree of physical dependence on nicotine. In this aspect, assessing the time between getting up and smoking the first cigarette of the day is the determining factor (Table 2). In general, it can be considered that smoking the first cigarette within the first 30min is a sign of a high degree of dependence.23,24 Nighttime smoking should also be taken into account in this group of smokers. If the subject wakens in the middle of the night in order to smoke, it is an unmistakeable sign of a high degree of dependence.
- (c)
Assessment of the degree of motivation and self-efficacy. For this purpose, it is useful to distinguish between those who indicate 8 or higher on the visual analogue scale and those who indicate lower scores. Depending on the score that the subject awards each of these variables, the intervention of the healthcare professional will be different. Thus, smokers with a high degree of motivation and self-efficacy will be prepared to receive treatment in order to quit smoking for good. In smokers with a low degree of motivation and high degree of self-efficacy, it will be necessary to intervene to improve their knowledge of the relationship between smoking and their disease. In smokers with high motivation and low self-efficacy, the offer of pharmacological treatment and psychological support is sufficient. Finally, in those with low motivation and low self-efficacy, the motivational interview is the treatment of choice.35,36
- (d)
Assessment of mood. This is very important information due to the impact that it could have on the therapeutic intervention. In subjects who answer yes to the questions stated above, the use of antidepressants will be necessary and consultation with a psychiatrist should be assessed; in those who respond negatively but who show a depressed mood, intense psychological support and continuous follow-up will be very useful. Those who do not have depression issues or depressed mood will find it easier to quit smoking.
Fig. 3 shows the diagnostic process for smoking in smokers with previously diagnosed COPD.
Treatment of Smoking in Smokers With Chronic Obstructive Pulmonary DiseaseThe decisions stated below on this topic are based on the strength of evidence graded according to the GRADE system.37
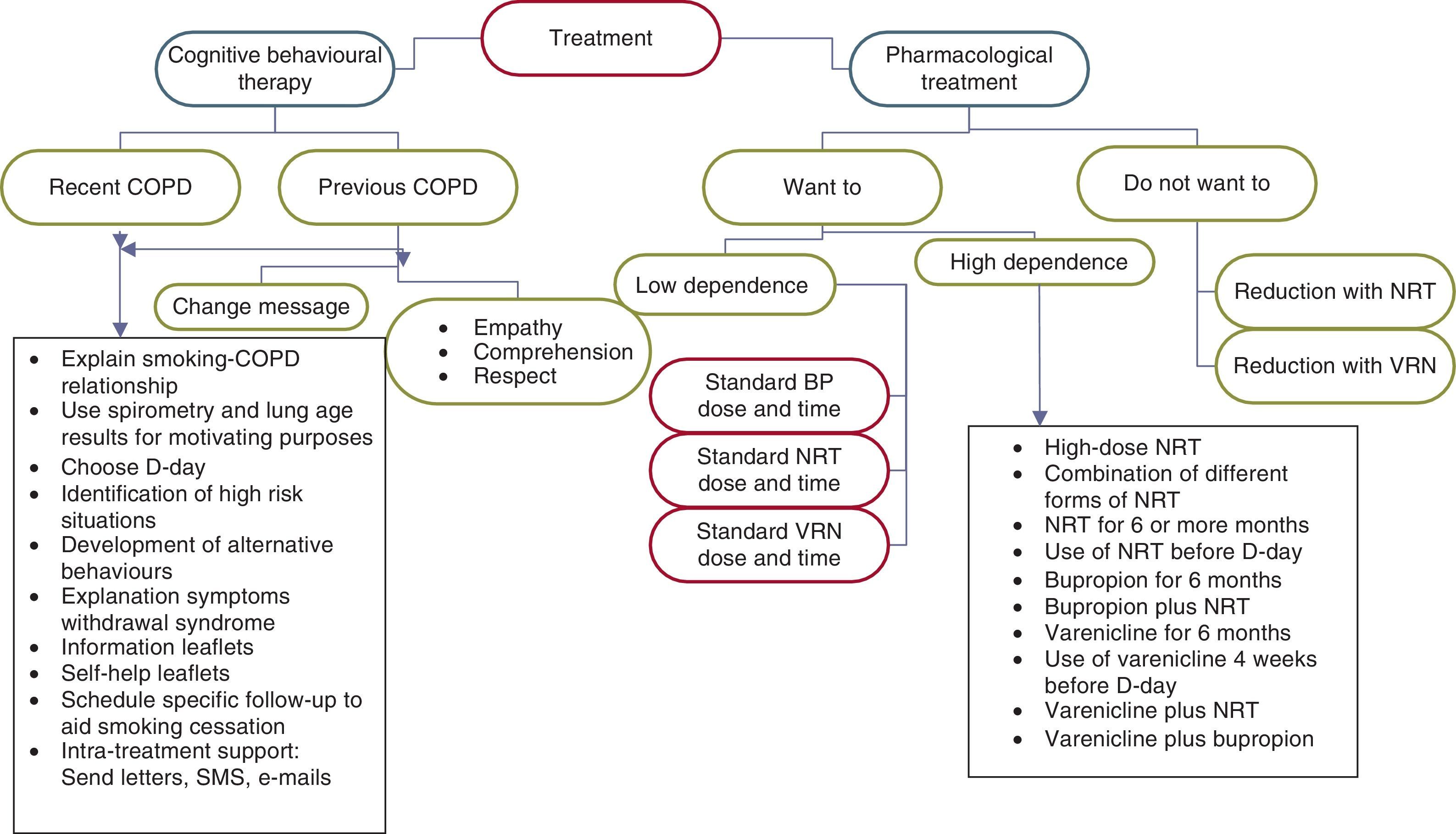
Treatment of smoking in smokers with COPD is made up of a combination of CBT and pharmacological treatment. The use of this type of combined treatment is a consistent recommendation with high quality evidence. Level of recommendation: strong.15,20,21,37,38
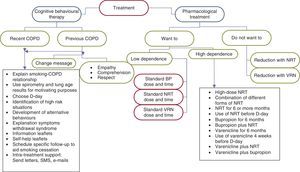
As in the case of the diagnosis, the characteristics of the CBT offered to these smokers should differ depending on whether they are smokers recently diagnosed or previously diagnosed with COPD (Fig. 4).
This therapy consists of the following aspects:
- (a)
Explanation of the close relationship between smoking and COPD. Subjects should be informed that smoking is the direct cause of their disease. They should also be warned about the following aspects: (1) that smoking cessation is the only therapeutic measure that has been shown to be effective for improving their disease; (2) that the use of pharmacological treatments for their disease will have very little effectiveness if they continue smoking; and (3) that, on the contrary, smoking cessation will be followed by a marked improvement in the course of their disease and the response of the COPD to treatment.
The lung age and spirometry results may be used for increasing the motivation to quit smoking in these patients. Some studies have found an increase in the motivation for cessation using both results.38–40 The lung age is related to the spirometric results. In normal conditions, there is a correlation between the lung age and chronological age of the subject. When the individual has COPD, their spirometric results correspond to a lung age much higher than their chronological age. Showing these results to patients and explaining their significance helps to increase their motivation to quit smoking.
- (b)
In smokers who are willing to make a serious attempt at smoking cessation, the intervention will be devoted to choosing the day to quit (D-day), the identification of high risk situations, development of alternative behaviours, explanation of the symptoms of withdrawal syndrome and its evolution, and the issue of leaflets with health information on smoking and COPD and self-help leaflets to quit smoking. In this group of smokers, scheduling follow-up visits with the sole objective of assessing the progress of the cessation process and monitoring the use of the different pharmacological treatments that have been prescribed for smoking cessation is a requirement that must be met in all cases. A follow-up schedule that is recommended in this patient group is as follows: first, second, fourth, eighth, twelfth, sixteenth, and twenty-fourth week after D-day.
It is important that the patient feels supported by the entire healthcare team during their smoking cessation process. It is crucial that they understand the importance of this for their health through the interest that the healthcare professionals who treat them place in their quitting. Telephone calls, sending personalised letters or even using new technologies (SMS messages, email messages, chats, etc.) may be useful for achieving this.
- (c)
In smokers who are not willing to make a serious attempt to quit at the present time, the need to quit smoking must be stressed at each of the visits that the subject makes to their doctor or nurse. The insistence should be made with empathy, cordiality and understanding, but with firmness and accompanied by offering all types of help.
The components of the CBT offered to these patients should be the same as those stated in the previous section. Nevertheless, it is important to take into account that the vast majority of these patients have already been warned by various healthcare professionals about the need for smoking cessation. For this reason, many of them will be reluctant to hear the “same message as always” from their doctor or nurse again. The correct health intervention on these patients requires not only that we change our message, but that we also change the way of presenting it. Therefore, the intervention in these patients should be carried out with empathy, respect and understanding, trying to increase the patient's motivation, self-efficacy and self-esteem. It should not be forgotten that depression is a common comorbidity in these subjects that may require specialised intervention. Any type of direct confrontation should be avoided. The patient should see the healthcare professional as a person willing to help them and able to understand all the problems that smoking is causing them.35,36
Occasionally, the determination of CO levels can indicate that the patient is smoking even when they deny that they are. The best way of confronting this situation is to explain to the patient the circumstances in which there may be false positives in the co-oximetry (Table 4) and alert them to avoid those circumstances, so that the next time that they attend the clinic and their expired air CO levels are measured, the results will be normal.35,36
Many of these patients are frustrated with the idea of making a new serious attempt at smoking cessation, since they have already tried on many occasions and have always failed. In this patient group, it is essential to send a new message: (a) it should be explained to the patient that there are new treatments and new ways to quit smoking; (b) it is very useful to analyse which treatments they used in past attempts and if these were used correctly; and (c) it is essential that they know that in a new attempt they will have the support of the entire healthcare team who are taking care of them.
Concern about weight gain after quitting smoking must be addressed. It is advisable to explain to the subject that when they quit smoking they may gain around 3–5kg in weight. Nevertheless, this gain should be controlled through moderate physical exercise and controlling the calorie intake.
Pharmacological Treatment of Smoking in Smokers With Chronic Obstructive Pulmonary DiseasePharmacological treatment of smoking in smokers with COPD is obligatory. The smoking characteristics in these types of smokers, and the imperative need that they have to quit smoking, require them to always use pharmacological treatment, and occasionally to do so intensively. The characteristics of pharmacological treatment of smoking that can be offered to these patients, depending on their motivation to make a serious attempt to quit, are discussed below.
Pharmacological Treatment of Smoking in Smokers With Chronic Obstructive Pulmonary Disease Who Want to Make a Serious Attempt to Quit SmokingIn this patient group, the recommended treatments are: nicotine replacement therapy (NRT), bupropion and varenicline.
Nicotine Replacement TherapyAll types of NRT have been shown to be effective and safe for aiding smoking cessation in these subjects in the different clinical trials that have been conducted.11–15,20
The use of this type of treatment is a consistent recommendation with high quality evidence.
Level of recommendation: strong.12–16,21,37 Some recommendations for the use of NRT are stated below. Taking into account that only nicotine gum, tablets and patches are available in Spain, the recommendations stated are restricted to these types of treatments only.
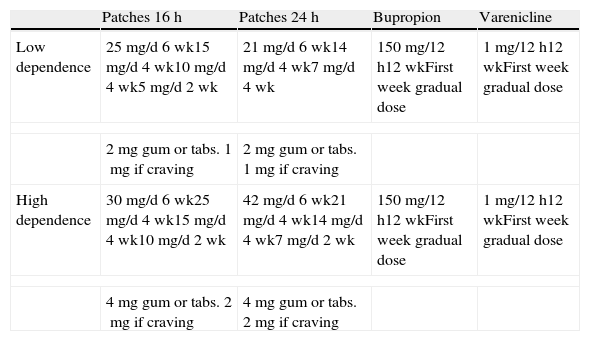
Table 5 shows the doses and time of use of the different types of NRT according to the degree of physical dependence on nicotine.11–15,19,20,28,35,41,42 Those who smoke fewer than 20 cigarettes per day, smoke their first cigarette 30min after getting up or who score 5 or fewer points on the Fagerström test are considered to have a low degree of dependence. On the contrary, those who smoke 20 or more cigarettes per day smoke their first cigarette within 30min of getting up or who score more than 5 points on the Fagerström test are considered to have a high degree of dependence. It is important to point out that in subjects who have used standard dose NRT and who have been unsuccessful, or in those in whom a standard dose does not control the symptoms of withdrawal syndrome, the NRT doses to be used are shown in the section on high degree of dependence.
Dose and Time of Use of Pharmacological Treatments According to the Degree of Dependence.
| Patches 16h | Patches 24h | Bupropion | Varenicline | |
| Low dependence | 25mg/d 6wk15mg/d 4wk10mg/d 4wk5mg/d 2wk | 21mg/d 6wk14mg/d 4wk7mg/d 4wk | 150mg/12h12wkFirst week gradual dose | 1mg/12h12wkFirst week gradual dose |
| 2mg gum or tabs. 1mg if craving | 2mg gum or tabs. 1mg if craving | |||
| High dependence | 30mg/d 6wk25mg/d 4wk15mg/d 4wk10mg/d 2wk | 42mg/d 6wk21mg/d 4wk14mg/d 4wk7mg/d 2wk | 150mg/12h12wkFirst week gradual dose | 1mg/12h12wkFirst week gradual dose |
| 4mg gum or tabs. 2mg if craving | 4mg gum or tabs. 2mg if craving | |||
tabs: tablets; d: day; h: hours; wk: weeks.
The main adverse effects of nicotine gum are oropharyngeal irritation, heartburn and temporomandibular joint pain. Those of the nicotine patches are pruritus and eczema at the site where the patch is placed, and myalgias and headaches as systemic effects. NRT has very few contraindications. Its use is only contraindicated in smokers with ischaemic heart disease of less than 4 weeks duration and in subjects with severe cardiac arrhythmias.41
A recent meta-analysis found that using standard dose nicotine patches for the 15 days prior to D-day was accompanied by an increase in their efficacy compared to when they were used with the usual regimen (OR: 2.17 [95% CI: 1.46–3.22).43 Nevertheless, this is a consistent recommendation with low quality evidence. Level of recommendation: weak.
BupropionThe use of this treatment in smokers is a consistent recommendation with high quality evidence. Level of recommendation: strong.16,17,37 Nevertheless, it should be taken into account that the results available only extend to 6 months of follow-up.
Table 5 shows the dose and time of use of bupropion according to the degree of physical dependence on nicotine.16,17,19,28,41,42 The main adverse effects of bupropion are insomnia, headaches, tremor and occasionally, mild skin hypersensitivity reactions. It is contraindicated in subjects with epilepsy or other convulsive disorders, in anorexia nervosa and in bulimia. It should be used with caution in subjects who are taking medicinal products that are metabolised in the liver via the cytochrome P450 pathway.41
In smokers who have previously used standard dose bupropion and who have been unsuccessful, or in those in whom standard dose bupropion does not control the symptoms of withdrawal syndrome, it is recommended to prolong the treatment up to 6 months; furthermore, the combination of bupropion with standard dose nicotine patches and/or gum may be recommended. Nevertheless, this is a consistent recommendation with moderate quality evidence. Level of recommendation: weak.19,28,37,41,42
VareniclineTo date, 2 studies have been conducted that show the efficacy and safety of the use of this medication to aid smoking cessation in these patients.18,19 The use of this type of treatment in these subjects is a consistent recommendation with high quality evidence. Level of recommendation: strong.18,19,37 Studies with varenicline show data up to 12 months of follow-up.
Table 5 shows the dose and time of use of varenicline according to the degree of physical dependence on nicotine.18,19,28,36 The main adverse effects of this drug are nausea and abnormal dreams. It does not have any absolute contraindications, except for severe renal failure.41
In smokers who have previously used standard dose varenicline and who have been unsuccessful, or in those in whom standard dose varenicline does not control the symptoms of withdrawal syndrome, it is recommended to prolong the treatment up to 6 months. This is a consistent recommendation with high quality evidence for this patient group. Level of recommendation: strong.19,28,37,44 In these patients with a high degree of dependence, combination with standard dose nicotine patches and/or gum may be recommended. Nevertheless, this is a consistent recommendation with very low quality evidence. Level of recommendation: weak.19,28,37,41,42,45 Another therapeutic possibility is the combination of varenicline with bupropion. A single, open-label, non-controlled study has been published, in which it was found that the combination of these 2 drugs is safe and may be followed by a slight increase in efficacy.46 The use of this combination is a consistent recommendation with very low quality evidence. Level of recommendation: weak.
In this group of smokers in whom the previous use of standard dose varenicline was unsuccessful, a new treatment regimen with varenicline may be used, consisting of the use of varenicline for 4 weeks prior to D-day, subsequently continuing for a further 12 weeks. This form of use was followed by a significant increase in the success rates at the end of treatment: 47.2% compared to 20.8%; P=.005.47 The use of varenicline in this way is a consistent recommendation with moderate quality evidence. Level of recommendation: weak.
Pharmacological Treatment of Smoking in Smokers With Chronic Obstructive Pulmonary Disease Who Do Not Want to Make a Serious Attempt to Quit Smoking at PresentIn this group, we must insist at all the follow-up visits on the need to make a serious attempt to quit smoking. The use of drugs like NRT and varenicline have been shown to be effective and safe in some studies for aiding smoking cessation in patients who, even if they do not want to do so at the time, are willing to reduce their consumption.11,48–51 Some advice for using NRT or varenicline in this subject group is discussed below.
Nicotine Replacement TherapyTo date, several meta-analyses have been carried out that show the efficacy and safety of using this medication to aid smoking cessation in patients who are not willing to make a serious attempt to quit smoking.48–50 The use of this type of treatment in these subjects is a consistent recommendation with high quality evidence. Level of recommendation: strong.18,48–50
VareniclineTo date, a single study has been carried out that shows the efficacy and safety of using this medication to aid smoking cessation in patients who are not willing to make a serious attempt to quit smoking.51 It was found that varenicline increased the attempts and motivation to quit smoking and a non-significant trend towards greater success of smoking cessation was observed.51 Taking these data into account, it is understood that the use of varenicline as treatment for the progressive reduction of smoking in smokers with COPD is a consistent recommendation with low quality evidence. Level of recommendation: weak.37,51
Conflict of InterestsThe authors declare that they have no conflict of interests.
Carlos Jimenez Ruiz has given talks and participated in studies with different medications for smoking cessation.
Please cite this article as: Jiménez-Ruiz CA, et al. Tratamiento del tabaquismo en fumadores con enfermedad pulmonar obstructiva crónica. Arch Bronconeumol. 2013;49:354–63.