Iatrogenic pulmonary embolisms are isolated complications that usually occur accidentally as a result of the migration of medical materials into the pulmonary vasculature. The devices most often associated with these events are central venous catheters (CVC) during an endovascular procedure.1,2
Nowadays, the number of diagnostic and therapeutic endovascular procedures has increased substantially and, consequently, the use of medical materials and devices has risen, contributing to the increased risk of pulmonary embolism. Embolisms associated with various procedures have been described, including vertebral cementation, coil placement, implantation of substances for esthetic purposes or to repair trauma. The most common foreign bodies are fragments of central lines or CVC, and sections of guidewires and catheters lost during an endovascular procedure.3–12 When loss or migration of a device is detected, recovery can be attempted using one of the many techniques currently available: catheters, loops, and other systems for recovering foreign bodies.1 The most frequently used are systems composed of a catheter, through which a guidewire forming a snare is inserted at its distal end.1
New social data systems are leading to the implantation of materials within the body. These devices work by communicating with other external systems, such as telephones. Their fundamental task is to offer the secure storage of information that is easy to access from the exterior, based on “near field communication” (NFC) technology, a system similar to that of bank cards that does not need a battery supply.
Both these devices and biointegrated sensors offer great possibilities for the future from multiple points of view.13,14 Biosensors, for example, are attracting attention in various scientific fields, particularly in medicine, as they are being developed to generate, transmit, and process biological signals, such as blood pressure, pulse, heart rate, blood glucose level, or respiratory signals.14
A percutaneous device is implanted via an introducer needle of a specific thickness into the back of the hand (anatomical pouch), between the first and second metacarpal. The aim is to implant a cylinder-shaped capsule measuring approximately 12mm×3mm (9 gauge) that would be lodged in or just under the subcutaneous cell tissue. The purpose of this device would be to record, store and transmit data to external devices in a secure and easily accessible way.
A recent publication15 describes the implantation of such a system that lost communication with the exterior. The possibility of implant migration was investigated and the biochip was finally located in the pulmonary artery. This form of pulmonary embolism caused by data recording/storage devices is new and differs from the presentations already described.
Since dorsal vein drainage of the hand flows into the cephalic vein with an eminently caudocranial trajectory, it cannot be ruled out in the case described that the implant had been positioned parallel to the path of the cephalic vein, and was integrated into the venous flow, facilitating its proximal migration to the pulmonary artery.
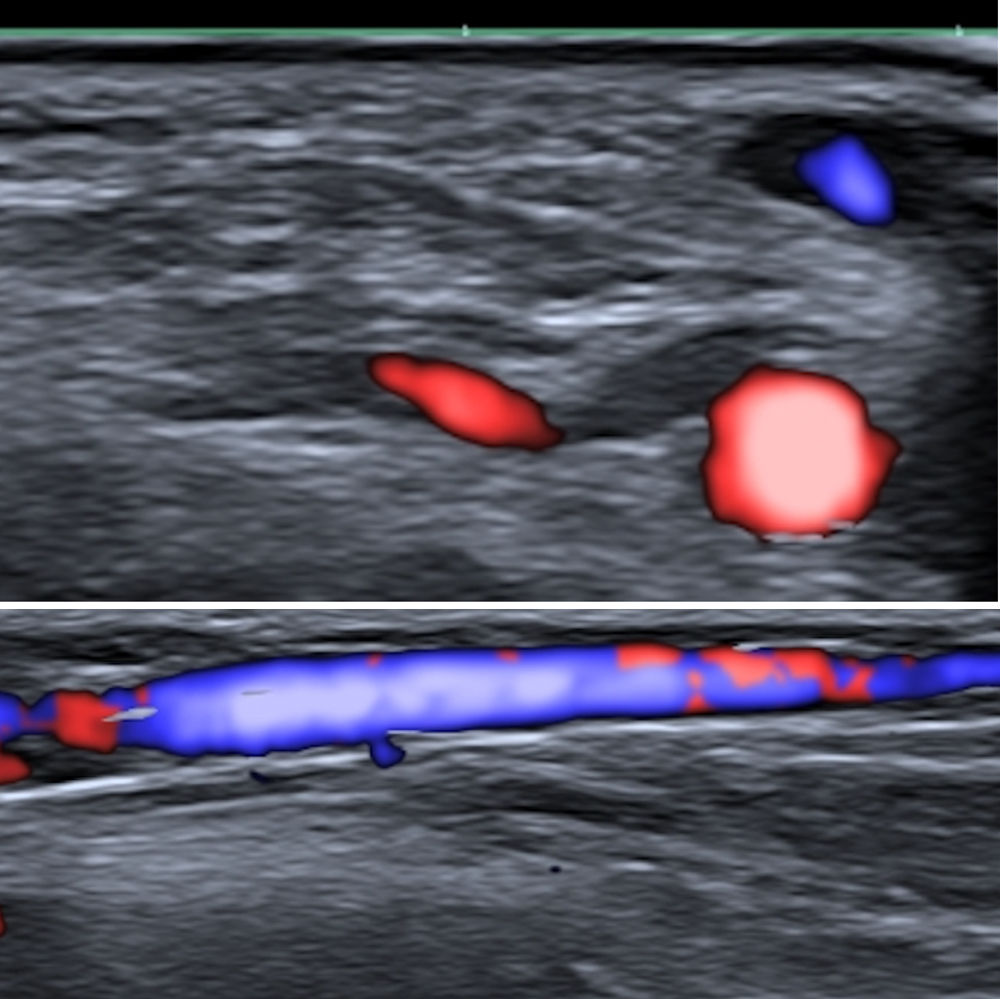
Fig. 1 shows the size of the cephalic vein and radial artery in the theoretical anatomical region of the implant (in a young man who we have taken as an example). As can be seen, both the artery and vein are large enough to be easily visible on ultrasound. This is obvious proof of the potential risks posed by these implants: they may become lodged in the vein, generating proximal migration, or they may cause local complications such as bleeding or hematoma due to venous (less likely due to the low blood pressure) or arterial (more likely because of the high pressure of the circuit) involvement.
Pulmonary embolisms are often difficult to diagnose because of their varied clinical presentation, the different nature of the embolized material, such as glues or coils, which constitute an important source of migrated materials, and the heterogeneous radiological signals they generate.8 As the use of these types of devices will presumably proliferate, there is likely to be an increase in the number of cases of pulmonary embolisms derived from implantation techniques. These procedures should be performed under medical supervision and should involve a mechanism for checking the extravascular location of the distal tip of the metal introducer prior to device release, including real-time ultrasound visualization. Ultrasound control may be difficult if proximal compression is not performed, given the low pressure of the venous system. As shown in Fig. 1, obtained under compression, both the vein and the artery are easily visible. This approach could ensure that the device is not placed intravascularly and that the vascular bed is unharmed; these precautions would without doubt increase the complexity of the implantation procedure but would clearly improve its safety. Another alternative would be to implant the device in a latero-lateral position, to avoid it following the main axis of the cephalic vein. It would be thus less likely to become lodged in a vein, substantially reducing or minimizing the risk of proximal pulmonary embolism.
FundingThis paper has not received any funding.
Conflict of interestsThe authors state that they have no conflict of interests.