Sedation during endobronchial ultrasound (EBUS) is essential due to the long duration of this procedure. We evaluated different models of sedation and their complications.
MethodA multicenter, prospective, observational study of 307 patients undergoing EBUS was conducted. Patients were sedated with: (a) midazolam bolus; (b) propofol infusion; (c) midazolam bolus and propofol infusion; (d) propofol infusion and remifentanil infusion, or (e) midazolam bolus and fentanyl bolus, and clinical variables were collected. Patients were asked to complete a satisfaction survey following the test.
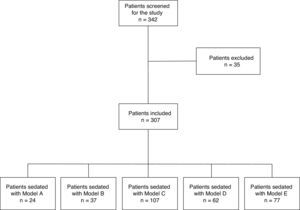
ResultsPatients per sedation model were: A 24, B 37, C 107, D 62 and E 77. Scores for perceived sensations of recall, pain, cough, dyspnea and prolonged examination (0.65±1.11, 0.3±0.73, 0.46±0.9, 0.29±0.73, and 0.59±0.96, respectively) were lower compared to fear and nervousness before the examination (1.26±1.37 and 1.5±1.41, respectively). High levels of indifference to repeating the procedure (1.49±1.3) and a reported pleasant feeling during the test (1.23±1.17), with low levels of anxiety (0.49±0.85) and discomfort (0.62±1.1), show that different models of sedation were well tolerated. Almost half the patients (46.6%) did not report any “worst moment” during the procedure, and 89.6% were willing to undergo a repeat test. The E and C models presented fewest complications (12.9% and 31.7%, respectively), and all were resolved with simple therapeutic measures.
ConclusionsThe models of sedation evaluated were well tolerated and most patients were willing to undergo repeat EBUS. Complications were few and easily resolved.
La sedación durante la ecobroncoscopia es importante debido a la duración prolongada de esta exploración. Evaluamos distintos modelos de sedación y sus complicaciones.
MétodoSe realizó un estudio multicéntrico, prospectivo y observacional en el que recogieron distintas variables en 307 pacientes con distintos modelos de sedación: a) midazolam en bolo; b) propofol en perfusión; c) midazolam en bolo y propofol en perfusión; d) propofol en perfusión y remifentanilo en perfusión, y e) midazolam en bolo y fentanilo en bolo. Finalizada la prueba, los pacientes contestaron una encuesta de satisfacción.
ResultadosLos pacientes por modelo de sedación fueron: A 24, B 37, C 107, D 62 y E 77. Las puntuaciones de las sensaciones percibidas de recuerdo, dolor, tos, disnea y exploración prolongada (0,65±1,11; 0,3±0,73; 0,46±0,9; 0,29±0,73; 0,59±0,96) fueron menores frente a miedo y nerviosismo antes de la exploración (1,26±1,37 y 1,5±1,41). Los valores elevados de indiferencia ante la repetición (1,49±1,3) y de sensación agradable de la prueba (1,23±1,17), junto con cifras bajas la sensación de angustia (0,49±0,85) e incomodidad de la exploración (0,62±1,1), muestran que los distintos modelos de sedación fueron bien tolerados. El 46,6% de los pacientes no encontraron ningún momento malo y el 89,6% se repetiría la prueba. Los modelos E y C fueron los que menos complicaciones presentaron (12,9 y 31,7%) y, en todos los casos, se resolvieron con medidas terapéuticas sencillas.
ConclusionesLos modelos de sedación analizados fueron bien tolerados y la mayoría aceptarían la repetición de la ecobroncoscopia. Las complicaciones fueron escasas y sencillas de resolver.
Endobronchial ultrasound (EBUS) is a minimally invasive technique that has a high yield in the diagnosis and staging of mediastinal disease and lung cancer.1,2 The ultrasound bronchoscope combines a flexible bronchoscope with a convex ultrasound transducer on the tip for simultaneous endoscopic vision and capture of ultrasound images of structures in contact with the bronchial wall. This modification results in an instrument with a thicker, rigid tip that must be inserted orally, an approach that is known to be more distressing for patients.3 Moreover, the endoscopic image is captured at a slightly different angle from a conventional bronchoscope because the optical system is not located on the tip of the instrument.
Bronchoscopy causes anxiety and intolerance in the patient, making it difficult for the bronchoscopist to perform their task. Performing the procedure in distressed patients reduces yield and can provoke hemodynamic and even respiratory changes. Sedation improves patient tolerance, allowing the bronchoscopist to work more easily,4 but the level of sedation applied can range from conscious sedation to general anesthesia, depending on the agents and the dose used. Conscious sedation is generally preferred, particularly for procedures requiring rapid recovery.3 However, in a recent study5 comparing the effect of light sedation and deep sedation on the number of lymph node territories aspirated and the histological yield of the samples, benefits were found for deep sedation. When sedation was deeper, patient tolerance was better, movements and cough during aspiration were reduced, and yield improved. Several groups recommend conscious sedation monitored by a bronchoscopist experienced in the use of drugs such as midazolam, propofol, and narcotics used alone or in combination, at low doses.6–8 Midazolam is an anxiolytic benzodiazepine that causes anterograde amnesia; propofol is a hypnotic agent with amnesic and antiemetic effects, and rapid onset and elimination, depending whether it is administered in intermittent boluses or by infusion. Narcotics – fentanyl and remifentanil – can be combined with either midozalam or propofol. They have useful analgesic and antitussigenic properties, and act in synergy, so the final dose of midazolam or propofol can be reduced.
Few groups have studied the level of sedation in endobronchial ultrasound, and the best drug or combination of drugs for this procedure remains undefined. Studies comparing patient satisfaction with different levels of sedation and the presence or absence of an anesthesiologist during the procedure are scarce.9 Studies that evaluate complications associated with the use of drug combinations during EBUS are also rare.
The objective of this study was to evaluate different sedation models and associated complications during EBUS, and to establish which is the safest drug or combination of drugs that gives most patient satisfaction.
Patients and MethodsStudy DesignThis was a multicenter, prospective, observational study comparing 5 different sedation models. It was approved by an ethics committee, and all patients enrolled signed informed consent forms and agreed to the use of data collected during the procedure. Sedation data were recorded during 342 consecutive EBUS performed between February 2011 and June 2012. The bronchoscopy units of 3 Spanish hospitals participated: Hospital Universitario y Politécnico La Fe, Valencia, Hospital Germans Trias i Pujol, Badalona and Hospital Fundación Jiménez Díaz, Madrid. Endoscopies were performed in all cases by pulmonologists with experience in EBUS techniques. In addition to the endoscopist, the team comprised another pulmonologist who obtained the aspirates, and a nurse and nursing auxiliary (or 2 nurses) who processed the samples and monitored the patient. For procedures involving propofol infusion and combined propofol and remifentanil infusion, an anesthetist was present to monitor sedation.
Study PatientsA total of 342 patients without contraindications for sedation were included in this study. All patients were evaluated before the procedure by a nurse with experience in sedation and a pulmonologist or anesthetist, and their physical status was classified according to American Society of Anesthesiologists (ASA) criteria. Exclusion criteria were: allergy to any of the sedative agents; ischemic heart disease or hemodynamic instability (defined as heart rate <60 or >120bpm, or systolic pressure <100 or ≥180mmHg); severe (defined as FEV1 ≥30% and <50% predicted post-bronchodilator) or very severe COPD (defined as FEV1 <30% predicted post-bronchodilator or <50% with chronic respiratory failure); kidney or liver failure, according to laboratory test results; electrolyte imbalance; mental or neurological disease; drug or alcohol dependence; or lower respiratory infection with respiratory failure.
Procedure and ProtocolData were collected from 5 sedation models: Model A, midazolam bolus; Model B, propofol continuous iv infusion; Model C, midazolam bolus and propofol infusion; Model D, propofol and remifentanil infusion; and Model E, midazolam bolus and fentanyl bolus. An anesthetist monitored sedation in Models B and D. In all cases, patients received the standard level of sedation used for performing endobronchial ultrasound. If any other model was used, this was at the criteria of the pulmonologist and the anesthetist. All patients were monitored for heart and respiratory rates, oxygen saturation, and blood pressure. Transcutaneous pCO2 and depth of sedation with bispectral index (BIS) were monitored in units equipped with these devices. Patients received supplemental oxygen, increasing when oxygen saturation fell below 90%. Venous access was maintained for the infusion of sedatives and, if necessary, for reversing the effect of these drugs or for managing possible complications. Before the patient was sedated, local anesthesia in the form of 10% lidocaine was administered in the oropharyngeal region, mostly topically but on occasion by tracheal cricothyroid membrane puncture. A mouthpiece was inserted to protect the ultrasound bronchoscope, and a modified Guedel tube was used to assist patient ventilation. Depth of sedation was monitored by BIS10 or the sedation visual analog scale (VAS). The sedation VAS consists of a scale scored from 0 to 10, in which 0 represents absence of sedation, 2 is minimum sedation (defined as normal responses to verbal stimuli), 5 is moderate sedation (defined as brisk, purposeful response to verbal or tactile stimulus), 8 is deep sedation (defined as purposeful response to painful or repeated verbal, tactile stimulus), and 10 is general anesthesia (defined as patient who cannot be woken, despite painful stimulus). Monitored variables, medication, degree of sedation, and possible complications were recorded on a specially designed worksheet, before, every 10min during, and every 20min after completing the procedure until the patient was discharged from the recovery room (Appendix A).
Complications were defined as oxygen saturation <90%, rise or fall in systolic or diastolic blood pressure >30% of baseline level, bradycardia <50bpm or tachycardia >120bpm, bradypnea <8 breaths per minute, or tachypnea >22 breaths per minute.
Endobronchial ultrasound was performed with a BF-UC 180F ultrasound bronchoscope (Olympus, Tokyo, Japan) and ultrasound images were processed using an EU-ME1 processor (Olympus, Tokyo, Japan). A 22-gauge needle was used for aspiration.
One hour after the procedure, if they had recovered from the effects of sedation, patients completed a questionnaire about different aspects of their perception of the examination. The questionnaire consisted of 14 multiple-choice questions. In 12, patients graded their response on a Likert scale (4=a lot, 3=quite a lot, 2=a bit, 1=a little, and 0=very little) (Appendix A) and in 2 they were asked to choose between different possible answers.
Statistical AnalysisQuantitative variables were presented as mean±standard deviation, and qualitative variables as absolute value and percentage of total. The Kolmogorov–Smirnov test was used to analyze the normal distribution of the variables. Comparison between baseline variables of the treatment groups was performed using the ANOVA test, and Student–Neuman–Keuls and Tukey's HSD tests were used for the post hoc subgroup analysis. Statistical significance was set at P<.05.
ResultsDuring the study period, 342 consecutive patients who had undergone endobronchial ultrasound completed the questionnaire. Thirty-five questionnaires were excluded because they were incomplete (Fig. 1). The remaining 307 questionnaires were completed by 226 (73.6%) men with a mean age of 63.4±12.4 years (range 29–87 years), and 81 women, mean age 61.47±11.8 years (range 37–85 years). Mean duration of the procedure was 45.27±16.9min (range 15–90min). Twenty-four patients received Model A sedation, 37 received Model B, 107 received Model C, 62 received model D, and 77 received model E. There were no statistically significant differences in patient age among the sedation models. The duration of the procedure differed significantly among the different models (P<.001), and the post hoc analysis revealed 2 homogeneous subgroups, namely A, C and E, versus E, B, and D (P=.82) (Table 1). Reasons for performing EBUS were: study of mediastinal lymphadenopathies (156 cases); staging (71); simultaneous lymphadenopathy study and staging (35); study of mediastinal (22) and hilar (9) mass; and other indications (14). Sedation Models A, C and E were performed without the presence of an anesthetist. The level of sedation of all patients was monitored by at least 1 of the available systems, most often the VAS (62.1%). Model C was the only regimen monitored with BIS.
Age (years) and Duration (min) of Test by Groups, Expressed as Mean±SD. P-value Calculated With Post Hoc Test.
| Model An=24 | Model Bn=37 | Model Cn=107 | Model Dn=62 | Model En=77 | Total | |
|---|---|---|---|---|---|---|
| Age (range) | 63.59±13.65(42–87) | 66.24±10.40(34–82) | 61.75±11.84(29–85) | 63.81±12.5(34–83) | 62.64±12.53(33–85) | 62.9±12.3(29–87) |
| Men | 21 | 31 | 79 | 43 | 52 | 226 |
| Women | 3 | 6 | 28 | 19 | 25 | 81 |
| Duration of sedation (range) | 34.86±13.36(18–68) | 56.2±19.1(18–90) | 54.22±11.74(35–75) | 56.34±17.52(15–90) | 45.35±12.1(20–70) | 45.27±16.9(15–90) |
| P-value | ||||||
| Model A | – | .0001 | NS | .0001 | NS | |
| Model B | .0001 | – | .0001 | NS | .005 | |
| Model C | NS | .0001 | – | .0001 | .002 | |
| Model D | .0001 | NS | .0001 | – | .0001 | |
| Model E | NS | .005 | .002 | .0001 | – | |
NS, not significant; SD, standard deviation.
Patients’ perception of the various aspects of the procedure, and scores for each Likert-type question are shown by sedation model in Table 2. (Responses to the other 2 questions – what was the worst moment of the procedure and if they would repeat the EBUS – are shown in Table 3.) Patients’ response scores – overall and by sedation model – are given in Table 2. Overall scores for each question show that patient-perceived sensations of recall, pain, cough, dyspnea, and prolonged procedure (0.65±1.11, 0.3±0.73, 0.46±0.9, 0.29±0.73, and 0.59±0.96, respectively) are scored lower than the sensations of fear and nervousness before the procedure (1.26±1.37 and 1.5±1.41, respectively). The higher scores given to indifference toward repetition (1.49±1.3) and pleasant sensation during the procedure (1.23±1.17), together with low scores for sensation of distress (0.49±0.85) and discomfort (0.62±1.1) during the procedure indicate that the EBUS was well tolerated with the various sedation models. When we compare the responses to each question by sedation model, significant differences are found for each question among the various models. Post hoc tests for perception of cough, pain, dyspnea, and recall revealed 2 significant homogeneous groups formed by sedation Models A and E, and Models B, C, and D. Two groups were also obtained for other parameters, consisting of Model E versus the other sedation models.
Likert Scale Response Score (4=a lot, 3=quite a lot, 2=a bit, 1=a little, 0=very little) for the Questions Posed to Patients After Endobronchial Ultrasound, by Sedation Model, Expressed as Mean±SD.
| Model An=24 | Model Bn=37 | Model Cn=107 | Model Dn=62 | Model En=77 | Total | |
|---|---|---|---|---|---|---|
| Recall | 1.79±1.28 | 0.33±0.72 | 0.25±0.64 | 0.1±0.39 | 1.35±1.2 | 0.65±1.1 |
| Pain | 0.7±1.15 | 0.06±0.33 | 0.1±0.41 | 0.01±0.01 | 0.84±1.01 | 0.3±0.73 |
| Cough | 1.04±1.43 | 0.03±0.17 | 0.33±0.83 | 0.21±0.7 | 0.92±0.98 | 0.46±0.9 |
| Feeling of lack of air | 0.7±1.36 | 0.03±0.17 | 0.4±0.79 | 0.05±0.28 | 0.77±0.97 | 0.29±0.73 |
| Prolonged procedure | 0.92±1.02 | 0.5±1.06 | 1.41±1.45 | 0.31±0.65 | 1.1±1.16 | 0.59±0.96 |
| Fear before the procedure | 1.71±1.23 | 0.64±1.12 | 1.64±1.44 | 1.03±1.14 | 1.76±1.39 | 1.26±1.37 |
| Nervousness before procedure | 1.33±1.61 | 0.94±1.14 | 0.63±1.07 | 1.06±1.23 | 2±1.40 | 1.50±1.41 |
| Fear of repeating procedure | 0.54±1.18 | 0.56±0.84 | 0.75±1.08 | 0.48±1.08 | 1.68±1.32 | 0.83±1.21 |
| Nervous of repeating procedure | 0.79±1.14 | 0.83±1.01 | 1.51±1.36 | 0.81±1.04 | 1.83±1.3 | 1.04±1.25 |
| Indifference to repeating the procedure | 1.21±1.21 | 1.42±1.32 | 0.35±0.58 | 1.84±1.44 | 1.25±1.06 | 1.49±1.3 |
| Sensation of distress | 0.54±0.88 | 0.11±0.40 | 1.32±0.62 | 0.13±0.46 | 1.2±1.11 | 0.49±0.85 |
| Discomfort of the procedure | 0.88±1.19 | 0.46±1.72 | 0.41±0.65 | 0.27±0.7 | 1.25±1.12 | 0.62±1.1 |
| Pleasant feeling during the procedure | 0.85±0.81 | 1.63±1.21 | 1±0.99 | 1.76±1.28 | 0.92±1.08 | 1.23±1.17 |
| Total | 1±1.19 | 0.58±0.86 | 0.78±0.91 | 0.62±0.8 | 1.30±1.16 |
Expressed as mean±standard deviation.
Responses to Questions: “What was the worst part for you?” and “Would you repeat the endobronchial ultrasound, if necessary?” Questions Posed to Patients After the Endobronchial Ultrasound, by Sedation Models. Data Expressed as Frequencies (%).
| Model An=24 | Model Bn=37 | Model Cn=107 | Model Dn=62 | Model En=77 | Mean (%) | |
|---|---|---|---|---|---|---|
| What the worst part? | ||||||
| When I received the anesthesia | 7.3 | 0 | 31.7 | 18.5 | 1.4 | 11.78 |
| When they inserted the ultrasound bronchoscope into my mouth | 23.8 | 32.8 | 10.2 | 2.8 | 30.9 | 19.94 |
| The beginning of the procedure | 20.2 | 0 | 3 | 4.2 | 14.3 | 8.34 |
| The end of the procedure | 12.1 | 21.6 | 6.3 | 4.2 | 15.5 | 11.9 |
| There was no bad part | 32.7 | 45.7 | 47.9 | 68.8 | 37.9 | 46.6 |
| It was all bad | 3.9 | 0 | 1 | 1.6 | 0 | 1.3 |
| Would you repeat the procedure, if necessary? | ||||||
| No | 4.2 | 0 | 2 | 1.6 | 1.3 | 1.82 |
| Probably not | 0 | 2.8 | 2 | 1.9 | 1.3 | 1.6 |
| Probably yes | 4.2 | 8.3 | 4.9 | 4.9 | 17.1 | 7.88 |
| Yes | 91.7 | 88.9 | 91.2 | 91.2 | 80.3 | 88.66 |
Expressed as mean±standard deviation.
When asked about the worst moment during the procedure, 46.6% of patients did not report any bad moment during the procedure, and the worst moments for the remaining patients were when the local anesthesia was applied to the upper airways (17.5%), and when the ultrasound bronchoscope was introduced into the trachea (15.4%) (Table 3). When asked if they would repeat the examination, 89.6% answered that they would be willing, if necessary.
Complications occurring during the EBUS procedure are listed in Table 4. Models E and C produced fewer complications (87.01% and 68.2%, respectively, of complication-free procedures). A higher percentage of patients had complications with Models B and D (86.5% and 69.4%, respectively). All complications were resolved with simple therapeutic measures taken in the endoscopy room.
Patients With No Complications and Percentage of Patients With No Complications, and Complications Occurring During Endobronchial Ultrasound, by Sedation Models, Expressed as Absolute Number and Percentage (%) by Sedation Models.
| Model An=24 | Model Bn=37 | Model Cn=107 | Model Dn=62 | Model En=77 | Total | |
|---|---|---|---|---|---|---|
| Patients with no complications | 14 (58.3) | 5 (13.5) | 73 (68.2) | 19 (30.6) | 67 (87.01) | 178 (57.9) |
| Desaturation | 1 (4.1) | 6 (16.2) | 4 (3.7) | 8 (12.9) | 4 (5.1) | 35 (7.5) |
| Hypertension | 8 (33.3) | 2 (5.4) | 11 (10.2) | 4 (6.4) | 1 (0.9) | 26 (8.4) |
| Hypotension | 0 | 9 (24.3) | 5 (4.6) | 18 (29.03) | 1 (1.2) | 33 (10.7) |
| Tachycardia | 1 (4.1) | 2 (5.4) | 0 | 1 (1.6) | 0 | 4 (1.3) |
| Bradypnea | 0 | 4 (10.8) | 1 (0.93) | 19 (30.6) | 0 | 24 (7.8) |
| Respiratory arrest | 0 | 0 | 0 | 2 (3.2) | 0 | 2 (0.6) |
| Cough | 4 (16.6) | 6 (16.2) | 11 (10.2) | 5 (8.06) | 1 (1.2) | 27 (8.7) |
| Other | 1 (4.1) | 4 (10.8) | 3 (2.8) | 5 (8.06) | 3 (3.8) | 13 (4.2) |
| All complications | 15 | 33 | 34 | 62 | 10 |
Our study compares different sedation models in patients undergoing EBUS with an ideally moderate level of sedation. All sedation methods were well accepted by the patients and the few complications that occurred were successfully managed in the endoscopy room.
Although the need for satisfactory sedation in bronchoscopy has been under discussion for some time,11 it is still a controversial area,12,13 partly because half of complications during bronchoscopy are a consequence of sedation.14 It is accepted practice to offer sedation, mainly in the form of a benzodiazepine, such as midalozam, to patients who request it,15 but some groups systematically use sedation,16 unless it is contraindicated. EBUS, however, with its oral approach, is a more complicated and less tolerated procedure that generates greater patient discomfort. Appropriate sedation is essential for the bronchoscopist to be able to complete the examination and for the patient to tolerate it.
International guidelines, such as BTS15 or ACCP,17 have established recommendations for bronchoscopy and ultrasound bronchoscopy procedures. However, they do not provide any clear criteria for the degree of sedation necessary or which drugs to use. A combination of 2 sedative agents has been proposed as a safe option for patients undergoing diagnostic EBUS.9 However, very few studies have evaluated the best route of administration or the safest method of monitoring sedation. In most pulmonology departments, the most commonly used combination for bronchoscopy is probably fentanyl and midazolam, which is why it is also the most commonly used in EBUS, and why we included it in our analysis. Other groups have introduced propofol, either in boluses or continuous infusion. Sarkiss et al.18 used intravenous propofol in EBUS, and did not encounter any significant complications. When we compared cough, pain and dyspnea among our patients who received the different regimens, we found that the model that included propofol was more successful in controlling these symptoms than those using midazolam. Thus, while the degree of sedation of our patients was similar, irrespective of the method used to determine it, propofol provided better symptom control.
When patients sedated and not sedated during bronchoscopy are compared, patients who do not receive sedation are more reluctant to repeat the test.4 In our patients, another of the benefits of using sedation for performing EBUS was that 89.6% of the patients were willing to repeat the procedure if necessary, irrespective of the type of sedation administered. These findings coincide with those of Steinfort and Irving.19 Sedative-induced lack of recall following the procedure is one of the variables associated with the patient's willingness to repeat it. Moreover, 46.6% of patients did not report any disagreeable moment, and the most poorly tolerated moments were when the upper respiratory tract was anesthetized and when the bronchoscope was inserted. Again, this finding reflects those of other studies.4 Sedation begins before local anesthesia is applied and the ultrasound bronchoscope is introduced, but the bronchoscopist may have to initiate the procedure before the patient is fully sedated, as some degree of patient collaboration is required for the local anesthesia. The level of sedation measured by BIS before starting the EBUS also deepens when the ultrasound bronchoscope is inserted in the airway.
The availability of several sedation models is due, in part, to the potential adverse effects of the drugs used. Complications occurred with all the sedation models analyzed in this study, all of which were resolved in the endoscopy room. The most common complication of sedation was hypotension, most frequently in patients receiving propofol and remifentanil infusion. Hypotension is one of the known side effects of propofol, so physicians using this agent must be skilled in managing this event. Cough is another common complication. Lower levels of local anesthesia are often used in sedated patients during endoscopy, and this sometimes leads to cough, both during and after completion of the procedure. Other complications included desaturation or bradypnea, possible complications in moderately sedated patients. We found that these complications were more common in the models which used propofol infusion and propofol with remifentanil. Remifentanil was not used by the pulmonologists in this study, and propofol infusion was used by only a few. This would explain why we found fewer complications in our study when the sedation was administered by the pulmonologist. In contrast, anesthetists are more accustomed to using different drugs and can find the best combination for the patient, according to the type of procedure and its duration. Remifentanil and propofol infusion have more potential adverse events, as we experienced.
The ability of the pulmonologist for managing these complications may be questioned, but in our opinion, this is not an issue: a pulmonologist's training includes mechanical ventilation, treatment of desaturation, and even the use of sedatives for ventilation in critical or intensive care units. Accordingly, in bronchoscopy units or interventional pulmonology, sedation is often administered by the pulmonologist. The models which presented fewest complications were E, C, and A, respectively, while the shortest durations were for Models A and E, respectively. The duration of the procedure under Model C was similar to that of Models B and D, but for B and D, the percentage of patients with complications was higher. Although the duration of the procedure differed significantly, we believe that the complications were due more to the choice of drug than to the length of the procedure, since the dose was adjusted according to the level of sedation at any given moment, and the duration of the procedure is unrelated to the percentage of complications.
Our study is limited insofar as we did not analyze the diagnostic yield of the EBUS according to the sedation model applied. However, EBUS yield for mediastinal staging and for the study of mediastinal lymphadenopathies and masses is well established, and is generally high,20 and we do not believe that this would differ among the different sedation models included in our study. Another limitation of the study is that the satisfaction of the bronchoscopists was not explored. Evaluating the presence of an anesthetist during the procedure was not a study objective, but it is unlikely that this factor has biased the results, since the responses of the patients regarding their perceptions were not influenced by the presence of an anesthetist.
The main conclusion of our study is that the different sedation models under study were all well tolerated by patients, to the extent that most patients would agree to repeat the procedure if necessary. In sedation models using bolus administration, patients were more poorly controlled, according to recall responses. Complications were rare and managed in the endoscopy room itself, so we are satisfied that some types of sedation can be administered by a bronchoscopist who is familiar with these drugs and capable of minimizing side effects.
Conflict of InterestsNone of the authors has any direct or indirect conflict of interest with the contents of this manuscript.
Please cite this article as: Cases Viedma E, Andreo García F, Flandes Aldeyturriaga J, Reig Mezquida JP, Briones Gómez A, Vila Caral P, et al. Valoración de la tolerancia y seguridad de 5 modelos de sedación durante la realización de la ecobroncoscopia. Arch Bronconeumol. 2016;52:5–11.