The seventh edition of the TNM classification, together with undeniable advantages, has limitations. The International Association for the Study of Lung Cancer (IASLC) Staging Committee has designed an international prospective study to improve this classification. A group of thoracic surgeons and pulmonologists was established in the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Oncology area, and created a registry of new lung cancer (LC) cases to participate in this project. The aim of this paper is to describe the main characteristics of the patients included.
Materials and methodsProspective, observational, multicentre, multiregional data collection (epidemiological, clinical, therapeutic and, especially, anatomical extension) study, according to the IASLC protocol, to analyse its prognostic value.
ResultsTwo thousand, four hundred and nineteen patients (83.6% men) from 28 hospitals were included. Ninety-six percent of the men and 54% of the women were smokers or ex-smokers. Chest/abdominal computed tomography (CT) scanning was performed in over 90% and positron emission tomography (PET)/CT scanning in 51.5% of cases. Among the 1035 patients who underwent surgery, 77% had early stages (ia to iib), and 61.6% of those treated using other methods had stage iv. Respiratory comorbidity was higher in men (47.9% versus 21.4%). The most common histological subtype was adenocarcinoma (34%), especially in non-smoking women (69.5%).
ConclusionsThe proportion of women and adenocarcinomas, as well as those resected at an early stage, increased among LC cases in Spain.
La 7.a edición de la clasificación TNM, junto a ventajas indudables, presenta limitaciones. El Comité de Estadificación de la International Association for the Study of Lung Cancer (IASLC) ha diseñado un estudio prospectivo internacional para perfeccionar esa clasificación. En el área de Oncología de la Sociedad Española de Neumología y Cirugía Torácica (SEPAR) se constituyó un grupo de cirujanos torácicos y neumólogos que creó un registro de nuevos casos de cáncer de pulmón (CP) para participar en ese proyecto. El objetivo del presente trabajo es describir las características principales de los pacientes incluidos.
Material y métodosEstudio prospectivo, observacional, multicéntrico y multirregional de recogida de datos (epidemiológicos, clínicos, funcionales, terapéuticos y, especialmente, de extensión anatómica), según protocolo de la IASLC, para analizar su valor pronóstico.
ResultadosSe incluyen 2.419 (83,6% hombres) pacientes de 28 hospitales. El 96% de los hombres y el 54% de las mujeres eran fumadores o exfumadores. Se practicó TC de tórax/abdomen en más del 90% y PET/TC en el 51,5% de los casos. Entre 1.035 pacientes sometidos a cirugía, el 77% tenían estadios tempranos (ia hasta iib), y de los tratados con otros medios, el 61,6% tenían estadio iv. La comorbilidad respiratoria fue mayor en hombres (47,9% frente al 21,4%). La estirpe más común fue adenocarcinoma (34%), especialmente en mujeres no fumadoras (69,5%).
ConclusionesLa proporción de mujeres y adenocarcinomas aumenta entre los casos de CP en España, así como los resecados en estadio temprano.
In 2010, 1 527 000 deaths1 were attributed to lung cancer (LC), one of the 10 major causes of death worldwide, and it seems very likely that this figure will increase in the coming years, given the trends observed in global tobacco consumption. In a large part of the Western world, a reduction in the specific mortality rate due to LC is being observed in men, including in Spain, although at a somewhat slower rate. Nevertheless, LC continues to be the principal cause of death from tumour disease in men and the third most common in women: 20 755 men and 3452 women in 2010, according to data from the Spanish National Institute of Statistics (INE).2
Despite advances in the knowledge of LC biology and the modest improvements in survival obtained with new drugs and new treatment regimens, LC remains a fatal disease in a high proportion of cases, and only patients diagnosed in the early stages have curative options with radical surgery or chemotherapy plus radiotherapy. Among the criteria for tumour classification, one of the most important is still the anatomical extension of the disease, expressed according to the familiar TNM classification, with its well-established and invaluable prognostic and treatment guidelines.
Since 1966, when the Union for International Cancer Control proposed the first TNM classification for LC, there have been several editions, each with its successive improvements and refinements. The 7th and latest version, drawn up and published by a committee of the International Association for the Study of Lung Cancer (IASLC) and adopted by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and other international societies, represented a substantial advance with respect to methodological rigour and the universality of the classification. In this project, unlike those of previous editions, not only patients undergoing surgery, but also all types of patients from all over the world, regardless of the treatment administered, were included.3,4 The SEPAR Bronchogenic Carcinoma Cooperative Group (GCCB) participated in this project with the inclusion of approximately 3000 patients, making important methodological contributions.5,6
Despite the improvements introduced in the 7th edition of the IASLC TNM, the authors recognised certain limitations, derived basically from the fact that the studies used as source data (clinical trials, institutional registries, patient series from various clinical settings) were not specifically designed for analysing the prognostic value of the degree of anatomical extension. For this reason, the IASLC Staging Committee drew up a protocol for prospective data collection, designed expressly for the validation and possible further refinement of the TNM classification. The protocol is long and detailed and is open to interested specialists around the world, who have been able to participate in the project by registering their patients in the IASCL international database which was set up and run by Cancer Research and Biostatistics7 (CRAB).
Although the data collection protocol is especially detailed with regard to anatomical staging, many other types of data are also collected (biological, laboratory and functional parameters, diagnostic procedures, tumour subtype, treatment administered and follow-up). For analysis of the prognostic value of the variables, survival time remains to be determined from the patient follow-up, which is still underway.
The aim of this article, now that the initial data collection phase has been completed, is to describe the most important findings derived from the patients included by our SEPAR (GCCB-II) group as part of the international IASLC project.
Materials and MethodsWhen the IASLC project was announced, a Coordinating Committee was organised by the SEPAR Oncology area, which, as of June 2007, contacted practically all the departments of our society, offering them the chance to participate in the project and help with the paperwork and authorisations required for participation. This registry project was accessible to all from the very beginning and, following the IASLC committee guidelines, allows the inclusion of any patient with a new diagnosis of histologically confirmed LC, from the date of entry in the study, regardless of the treatment given (including the option of palliative care only).
This is a prospective, observational, multicentre, data collection study of patients with LC, for participation in the international IASLC project aimed at producing the next TNM classification.
Database and VariablesThe international IASLC database, designed by the IASLC Staging Committee, is managed by CRAB and requires online entry of data in English. The vast majority of the variables are closed questions, some with mutually exclusive options, and others with concurrent multiple responses. This database provides explanatory documentation, comments and figures, and procedures for automatic detection of some errors and contradictions, in such a way that the user must correct any errors before proceeding to the next question. In addition, the database managers may request clarifications from the investigators if they have questions or detect inconsistencies.
In addition, as well as following the guidelines set down by the IASLC Committee, the GCCB-II group participants, after identifying some issues, decided to add some additional guidelines regarding the criteria for choice of values in some variables which could be ambiguous, with the aim of reaching the maximum consistency and precision possible.
The detailed protocol, available on the Internet,7 consists of a possible maximum of 619 variables for surgical cases and 403 for non-surgical cases. Although not all the variables with the possible values that can be assigned to each one can be detailed here, they can be summarised as follows: (1) General: affiliation; exposure to tobacco smoke; clinical, laboratory and functional parameters. (2) cTNM classification of LC, with a detailed description of the various classification criteria of the T, N and M components and specification of the tests and procedures carried out, according to certainty criteria. (3) Histological subtype with degree of differentiation and immunohistochemical results. (4) Modality and details of treatment administered. (5) pTNM, equally detailed for cases undergoing surgery. (6) Follow-up variables and vital status.
Statistical AnalysisComparison of means was carried out using the Student t-test. The χ2 test was used for comparison of percentages.
Although this was an observational study, the authorisation of an Ethics Committee was required from each of the participating centres.
ResultsAlthough the number of patients included by the end of 2012 was 2615, only the data recorded up to 30 September 2011 are presented, as the date on which sufficient data for this first description were available.
In the study, 2419 patients were included in 28 centres:
- (a)
12 hospitals registered only patients treated with surgery.
- (b)
10 hospitals included only patients without surgical treatment.
- (c)
6 hospitals included patients undergoing any treatment (surgical or other).
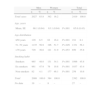
The characteristics of age, sex and smoking habit are shown in Table 1.
General Patient Characteristics.
| Men | Women | Total | |||||
| n | % | n | % | n | % | ||
| Total cases | 2027 | 83.8 | 392 | 16.2 | 2419 | 100.0 | |
| Age, years | |||||||
| Mean, SE | 66.1 (0.64) | 63.1 (0.64) | P<.001 | 65.6 (0.43) | |||
| Age distribution | |||||||
| ≤50 years | 129 | 6.5 | 62 | 16.4 | P<.001 | 191 | 8.1 |
| 51–70 years | 1135 | 56.9 | 196 | 51.7 | P=.029 | 1331 | 56.1 |
| >70 years | 729 | 36.6 | 121 | 31.9 | P=.053 | 850 | 35.8 |
| Smoking habit | |||||||
| Smokers | 965 | 48.0 | 131 | 34.1 | P<.001 | 1096 | 45.8 |
| Ex-smokers | 961 | 47.9 | 76 | 19.8 | P<.001 | 1037 | 43.4 |
| Non-smokers | 82 | 4.1 | 177 | 46.1 | P<.001 | 259 | 10.8 |
| Total | 2008 | 100.0 | 384 | 100.0 | 2392 | 100.0 | |
| No data | 19 | – | 8 | – | 27 | – | |
SE, standard error of the mean.
Table 2 shows the co-morbidity and performance status results, according to the IASLC protocol, distributed by sex. As can be observed, men had higher percentages of comorbidity for all diseases (except renal failure) and a greater incidence of weight loss and poor performance status.
Comorbidity and Patient Performance Status.
| Men | Women | Total | |||||
| n | % | n | % | n | % | ||
| Diabetes | 411 | 20.3 | 44 | 11.2 | P<.001 | 455 | 19.8 |
| Renal failure | 49 | 2.4 | 17 | 4.3 | P=.041 | 66 | 2.7 |
| Respiratory comorbidity | 971 | 47.9 | 84 | 21.4 | P<.001 | 1055 | 43.6 |
| Cardiac comorbidity | 882 | 43.5 | 129 | 32.9 | P<.001 | 1011 | 41.8 |
| Previous cancer | 383 | 18.9 | 65 | 16.6 | P=.2 | 448 | 18.5 |
| Alcoholism | 292 | 14.4 | 12 | 3.1 | P<.001 | 304 | 12.6 |
| PS (Zubrod scale)≥2 | 438 | 21.6 | 53 | 13.5 | P<.001 | 491 | 20.3 |
| Weight loss>5% of body weight | 476 | 23.5 | 72 | 18.3 | P=.027 | 548 | 22.7 |
PS, performance status.
With regard to the histological subtype (Table 3), epidermoid carcinoma was the most common type among men. However, adenocarcinoma is the most common tumour in women and overall. In section B of Table 3, the proportion of adenocarcinomas is compared as a function of smoking habit. It can be seen that this disease subtype is significantly more prevalent in subjects who never smoked, whether male or female.
Distribution According to Histological Subtype.
| Men | Women | Total | |||||
| n | % | n | % | n | % | ||
| (A) Distribution of tumour subtypes by sex | |||||||
| Adenocarcinomaa | 597 | 29.4 | 225 | 57.4 | P<.001 | 822 | 34.0 |
| Epidermoid | 755 | 37.2 | 48 | 12.2 | P<.001 | 803 | 33.2 |
| Small cell | 286 | 14.1 | 38 | 9.7 | P=.019 | 324 | 13.4 |
| Non-small cell, no further specification | 164 | 8.1 | 21 | 5.3 | P=.06 | 185 | 7.6 |
| Otherb | 225 | 11.1 | 60 | 15.3 | 285 | 11.8 | |
| Smokers and ex-smokers | Non-smokers | ||||
| n | % | n | % | ||
| (B) Distribution of tumour subtypes by sex according to smoking habit | |||||
| B1: Women | |||||
| Adenocarcinoma | 102 | 49.3 | 123 | 69.5 | |
| All other subtypes | 105 | 50.7 | 54 | 30.5 | P<.001 |
| B2: Men | |||||
| Adenocarcinoma | 551 | 28.6 | 42 | 51.2 | |
| All other subtypes | 1375 | 71.4 | 40 | 48.8 | P<.001 |
The number and percentage of the most common diagnostic tests and staging are shown in Table 4. Chest and upper abdomen CT scans and fibrobronchoscopy were carried out in nearly all patients. Among the tests performed for detecting possible metastasis, a PET or PET/CT was carried out in over 50% of all patients (whether they underwent surgery or not).
Diagnostic and Staging Tests Frequently Used.
| Type of test | n | % of total |
| Fibrobronchoscopy | 2223 | 91.9 |
| CT of chest and upper abdomen | 2367 | 97.8 |
| Bone scan | 407 | 16.8 |
| Head CT and/or MRI | 654 | 27.0 |
| PET or PET/CT | 1245 | 51.5 |
| Mediastinoscopy | 291 | 12.0 |
CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Distribution according to TNM staging (7th edition) is shown divided into two groups (Table 5): surgical patients (cTNM and pTNM) and non-surgical patients (cTNM). As can been seen, at the time of data analysis, insufficient data were available in some cases, so the figures presented do not reflect the total number of patients included.
Distribution by TNM Staging (IASLC 7th Classification).
| Stage | Patients treated with surgerya | Patients treated without surgery | ||||
| cTNM | pTNM | cTNM | ||||
| n | % | n | % | n | % | |
| ia | 309 | 36.2 | 123 | 17.5 | 16 | 1.2 |
| ib | 186 | 21.8 | 196 | 27.9 | 31 | 2.4 |
| iia | 116 | 13.6 | 139 | 19.8 | 20 | 1.5 |
| iib | 34 | 4.0 | 83 | 11.8 | 30 | 2.3 |
| iiia | 143 | 16.7 | 120 | 17.0 | 186 | 14.3 |
| iiib | 16 | 1.8 | 5 | 0.7 | 215 | 16.6 |
| iv | 50 | 5.8 | 37 | 5.3 | 299 | 61.6 |
| Total | 854 | 100.0 | 703 | 100.0 | 797 | 100.0 |
| Insufficient datab | 181 | – | 332 | – | 587 | – |
Finally, Table 6 provides a very brief summary of the therapeutic modality applied and, within the surgical option, the type of intervention.
Treatment Administered.
| Type of treatment | n | % of totala |
| Surgeryb | 1035 | 42.8 |
| CT and/or RTc | 1100 | 45.5 |
| Palliative care only | 284 | 11.7 |
| Type of surgery performed | n | % of total surgeries |
| Lobectomy | 709 | 68.5 |
| Bilobectomy | 47 | 4.5 |
| Pneumonectomy | 124 | 12.0 |
| Segmentectomy | 26 | 2.5 |
| Sleeve resection | 98 | 9.5 |
| Exploratory thoracotomy | 31 | 3.0 |
We would first like to point out that the patients included in this series, whether due to the study objective (analysis of prognostic factors for validation of the TNM classification) or the design (open study with inclusion of different patients depending on the centre: some surgical; others undergoing different treatments), are not and nor are they claimed to be a representative sample of all the epidemiological aspects of the LC patient population in Spain. However, we do think it is feasible and useful to compare some features with other similar papers with a view to making some inferences, particularly with regard to trends.
As already observed in some recent studies in Spain,8,9 the proportion of women with respect to the total number of LC cases is increasing rapidly. In the SEPAR GCCB registry from 1993 to 1997, the proportion of women was 7%6; in the Spanish Epicli-CP multicentre study, in 2003, it was 10.5%8; in a registry in the Spanish regions of Castilla-León and Cantabria carried out in 2007, it was 12.9%,10 and in a multicentre series of stage i surgical patients, it was 13%.11 In our registry, the proportion of women was 16.2%. These results are fully consistent with the LC mortality trends recorded by the INE,2 which show a clear increase in LC deaths in women compared to a stabilisation in men. Despite this trend, attributable to the increase in tobacco use among women, the percentage of non-smokers among women with LC (46.1%) is very high and much greater than that among men (4.1%), although it is less than that recorded among women with LC in the Epicli-CP study (68%).8 If the smoking trends in Spain (32.5% of smokers in the male population and 22.2% of females, according to the National Health survey in 2009) are taken into account, particularly with regard to the increase in female smokers, a continued increase in the rate of LC among women in the coming years can be predicted.
Since LC usually occurs in elderly smokers and ex-smokers, the high rate of comorbidities, particularly of the cardiorespiratory type, is unsurprising. In our cases, the incidence of both cardiac-related and respiratory concomitant diseases was greater than 40% (Table 2). A large part of this comorbidity is attributable to long-term smoking. For this reason, it is understandable that men with a diagnosis of LC (96% of whom are smokers or ex-smokers) have a greater percentage of comorbidities than women (54% among smokers and ex-smokers). This higher incidence of comorbidities may also explain, at least in part, why males have a poorer performance status at the time of diagnosis. Given the differences in this respect in the data collection protocols of the different publications, it is difficult to draw comparisons.9 In a large series from the Mayo Clinic, the authors report COPD in 32.9% and heart disease in 11.1%12; in the GCCB group registry between 1993 and 1997, the authors observed that 50% of their cases had COPD,13 a somewhat higher figure, but not very different from ours.
With regard to tumour subtype, adenocarcinoma is the most common form in most countries and its incidence appears to increase in younger cohorts.14–16 In Spain and in some other countries in the south of Europe, perhaps due to the smaller proportion of women with LC compared to other regions, epidermoid carcinoma has been reported as the most common.9 In our study, we found for the first time, in a multiregional study in Spain, a higher rate of adenocarcinomas in the overall patient set. This trend is influenced without doubt by the growing proportion of women. As can been seen in Table 3, up to 69% of the women with LC who never smoked had adenocarcinoma, but even among smokers and ex-smokers this percentage was over 50%. Epidermoid carcinoma is still predominant among men, but when the small group of non-smokers is examined, adenocarcinoma, with 51.2%, represents the majority. In Castilla-León and Cantabria, although the percentage of this tumour subtype was lower among women, the same difference in histological distribution depending on smoking habit was observed.10
With regard to staging, the 7th edition of the TNM classification3,4 was used for this study. The distribution of stages is presented separately for patients undergoing surgery and those receiving other treatments, since, as mentioned above, our overall patient set is not representative of the Spanish LC population. Indeed, given the proportion of hospitals that provided only surgical patients, it was to be expected that the complete set would show marked over-representation of surgical cases, as has happened (Table 6). Moreover, comparison of the results with other papers, even if analysed by subgroups (surgical patients versus non-surgical patients), is difficult, since many of the publications used the previous TNM classification (6th edition, Mountain).
Even with these limitations and the necessary reservations, grouping stages into 3 categories (local, locoregional and metastatic), in the same way as the US epidemiological cancer surveillance group (SEER) distributed their data,17 it is worth pointing out that local LCs (stage ia-iib) in our series account for 77% of those undergoing surgery, a higher percentage than that recorded by the GCCB group between 1993 and 1997 (around 60%)6 and very similar to that obtained by the Icelandic national registry of patients undergoing interventions between 1994 and 2008 (78.7%).16 It appears, at least in Spain, that among operated patients, there is a growing proportion of patients presenting with early stages, compared to locally advanced stages (iiia and iiib). In accordance with this, the percentage of pneumonectomies with respect to the total number of surgical procedures has fallen: 29.5% in the GCCB registry of 1993-199718 and 12% in the current series (Table 6). However, in the above-mentioned Icelandic study, when the period under study (1994–2008) was divided into three stages, with the aim of detecting trends, the authors did not detect any changes either in the proportion of early stages or in the number of pneumonectomies performed: 12.9% in 1994–1998 and 16.1% in 2004–2008. Although this difference in trends may be due to other reasons, it should be pointed out that PET was not performed in any of the cases during the period examined by these authors.16
With regard to patients who received other treatments (chemotherapy, radiotherapy or palliative care only), the proportion of cases with stage iv disease (61.6%) is higher than in the majority of studies of series around the world: 27% in Slovenia in 199619; 41% in Spain (Epicli-CP study, 2003)8; 34.2% in the Mayo Clinic experience 1997–200312; and 56% in the US epidemiological surveillance registry.17 The higher percentage observed in our series may be explained in part by the fact that we excluded surgical patients from this group. Moreover, the criteria for participation and inclusion in our registry limited comparability with other global LC studies, but we would like to point out that, unlike most large series, in ours, given the central objectives of the study (a precise TNM evaluation), special emphasis has been placed on more exact staging tests, as can be seen in Table 5. In fact, PET and PET/CT were performed in more than half of the patients. It is quite possible that this has contributed to the detection of a not insignificant subgroup of cases with stage IV disease and silent metastases which would have gone unnoticed with less exhaustive staging procedures.
We hope that the detailed analysis of the data recorded by our group, together with those from other countries which have contributed and continue to contribute to the international IASLC project, allows us, once the patients’ survival time has been determined, to continue our advances towards the common objective of achieving an even more robust and precise TNM classification. Nevertheless, without in any way taking away from the importance of this classification of anatomical disease extension, we are aware that this is only one more aspect of the tumour disease and a consequence of its biological properties, the molecular substrate of which is only just beginning to be revealed. For this reason, we have reached agreements with other Spanish research groups, which have the necessary resources for participating in projects for the genomic and molecular analysis of resected tumour samples. We hope that, in this way, the molecular information can be combined with the detailed clinical and anatomical extension data collected from our patients.
Conflict of interestsThe authors declare no conflict of interests.
José Abal Arca and Isaura Parente Lamelas, Respiratory Medicine, Complejo Hospitalario de Ourense.
Pablo León Atance, Chest Surgery, and Ana Núñez Ares, Respiratory Medicine, Complejo Hospitalario de Albacete.
Luis Miravet Sorribes, Respiratory Medicine, Hospital La Plana, Vila Real, Castellón.
Ana Isabel Blanco Orozco, Chest Surgery, y M. Ángeles González Castro, Respiratory Medicine, Hospital Virgen del Rocío, Seville.
Rosario Melchor Íñiguez, Respiratory Medicine, Fundación Jiménez Díaz, Madrid.
Luis García Arangüena, Respiratory Medicine, Hospital Sierrallana, Torrelavega, Cantabria.
Antonio Arnau Obrer and Ricardo Guijarro Jorge, Chest Surgery. Hospital General Universitario de Valencia, Valencia.
José Padilla Alarcón and Juan Carlos Peñalver Cuesta, Chest Surgery, Instituto Valenciano de Oncología, Valencia.
Manuel Mariñán Gorospe, Chest Surgery, Hospital San Pedro, Logroño.
Esther Fernández Araujo, Chest Surgery, and Felipe Andreo García, Respiratory Medicine, Hospital Universitario Germans Trias i Pujol, Badalona, Barcelona.
Gloria Francisco Corral, Respiratory Medicine, and Sara Cerezo González, Oncología, Hospital La Mancha Centro, Alcázar de San Juan, Ciudad Real.
Guillermo González Casaurrán, Chest Surgery, Hospital Gregorio Marañón, Madrid.
Sara Naranjo Gozalo and Carlos Álvarez de Arriba, Chest Surgery, Hospital Marqués de Valdecilla, Santander.
Manuel Núñez Delgado, Respiratory Medicine, Hospital de Meixoeiro, Vigo, Pontevedra.
M. Teresa González Budiño, Respiratory Medicine, Hospital General Universitario, Oviedo, Asturias.
Francisco Abad Cavaco, Respiratory Medicine, Hospital la Fe, Valencia.
Ramón Magaroles and Leonardo de Esteban Júlvez, Respiratory Medicine, Hospital Joan XXIII, Tarragona.
M. José Pavón Fernández, Respiratory Medicine, Hospital Severo Ochoa, Leganés, Madrid.
José Antonio Gullón Blanco, Respiratory Medicine, Hospital San Agustín, Avilés, Asturias.
Beatriz de Olaiz Navarro, Chest Surgery, Hospital de Getafe, Getafe, Madrid.
Ignacio Escobar Campuzano and Iván Macía Vidueira, Chest Surgery, Hospital de Bellvitge, L’Hospitalet de Llobregat, Barcelona.
Santiago García Barajas, Chest Surgery, Hospital Infanta Cristina, Badajoz.
Jorge Herrero Collantes, Chest Surgery, Hospital Universitario Nuestra Señora de la Candelaria, Santa Cruz de Tenerife.
Jorge Freixenet Gilabert, Chest Surgery, Hospital Universitario Dr. Negrín, Las Palmas de Gran Canaria.
Alberto Saura Vinuesa, Respiratory Medicine, Hospital de Sagunto, Sagunto, Valencia.
Please cite this article as: Sánchez de Cos Escuín J, Serra Mitjans M, Hernández Hernández J, Hernández Rodríguez H, and other GCCB-II authors. Registro del Grupo Cooperativo de Cáncer de Pulmón-II de la Sociedad Española de Neumología y Cirugía Torácica. Estudio descriptivo. Arch Bronconeumol. 2013;49:462–467.
See Appendix 1.