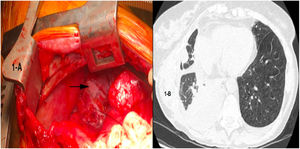
We present the case of a 64-year-old woman who had undergone single right lung transplantation for lymphangioleiomyomatosis (LAM), with no intraoperative or immediate postoperative complications. On postoperative day 7, pleural fluid drainage was milky in appearance, with exudative lymphocytic characteristics and triglycerides 630mg/dL, and she was therefore diagnosed with right chylothorax. Dietary treatment was established with enteral nutrition, medium chain triglycerides and octeotride for one week. However, given the persistence of the chylothorax (Fig. 1B), surgical treatment was indicated.
One hour before the surgical procedure, an oral solution of milk with butter was administered to enable macroscopic localization of the chylous fistula during the surgery. After dissection of the paraesophageal and periaortic space, a chylous fistula was located in the thoracic duct and its afferent vessels (Fig. 1A); these were ligated and hemostatic surface sealants were applied. The patient recovered well, with complete resolution of the chylothorax, and was in good health 12 months after the surgery.
LAM is a rare multisystem disease that predominantly affects women, and is classified as a low-grade neoplastic disease. It is characterized by progressive respiratory failure, recurrent pneumothorax, renal angiomyolipomas, and lymphatic disease (chylothorax, chylous ascites or lymphangioleimiomas).1,2
Radiologically, it is characterized by the presence of multiple round, thin-walled interstitial cysts.3 According to the European Respiratory Society, the diagnosis of LAM is established when characteristic radiological findings are present, associated with renal angiomyolipomas, chylothorax, chylous ascites, lymphangiomyolipoma or adenomegaly.4 In the absence of these, lung biopsy is recommended.
Lung transplantation is indicated in advanced stages of the disease, refractory to medical treatment, with 65% survival at 5 years post-transplantation, accounting for only 1% of the indications for lung transplantation according to the International Registry.5
Between 7% and 10% of patients with LAM develop chylothorax, which should be treated promptly since it leads to malnutrition, immunosuppression, respiratory and metabolic failure, and electrolyte imbalance, which can be fatal.6
In our case, the diagnosis was suspected after changes were noted in the macroscopic characteristics of the pleural drainage fluid, which coincided with the start of oral tolerance, and was confirmed by the determination of triglycerides in the pleural exudate. Treatment with a fat-free diet, medium chain triglycerides and octeotride was initiated to reduce gastrointestinal secretions and splanchnic blood flow.7,8 Sirolimus has also demonstrated effectiveness in the control of chylothorax,9 but was not used in our particular case due to risks in the healing process after recent surgery. However, conservative measures were not sufficient, suggesting the presence of a considerably large opening in the thoracic duct. For this reason, revision surgery was indicated.
When conservative treatment fails, as in the present case, invasive procedures such as surgery, chemical pleurodesis, bypass systems, or percutaneous lymphatic embolization are indicated.10 These procedures are considered when the chylothorax volume is greater than 1L/day for more than 5–7 days.
The surgical procedures described are ligation of the thoracic duct or, if not located, ligation of the fatty tissue between the aorta, vertebral bodies, esophagus, and azygos vein. In some cases, mechanical pleurodesis has been proposed as an equally effective method; however, in the present case, this procedure was ruled out, since the patient presented anatomical and morphological anomalies of the afferent lacteal vessels of the parietal pleura due to her underlying condition.
In summary, the presence of chylothorax in the postoperative period of lung transplantation for LAM initially requires early instigation of conservative, dietary and pharmacological treatment. If, despite these measures, the chylothorax is persistent or recurrent after reintroducing the enteral diet, surgical exploration is necessary. In our case, as a newly transplanted patient, sirolimus treatment was discouraged due to the high possibility of suture dehiscence following the recent lung transplant.
Please cite this article as: González García FJ, Baamonde Laborda C, Muñoz Fos A, Moreno Casado P, Redel Montero J, Algar Algar J, et al. Tratamiento quirúrgico de quilotórax postrasplante pulmonar por linfangioleiomiomatosis. Arch Bronconeumol. 2020;56:335–336.