The aim of this study was to develop a surgical risk prediction model in patients undergoing anatomic lung resections from the registry of the Spanish Video-Assisted Thoracic Surgery Group (GEVATS).
MethodsData were collected from 3533 patients undergoing anatomic lung resection for any diagnosis between December 20, 2016 and March 20, 2018.
We defined a combined outcome variable: death or Clavien-Dindo grade IV complication at 90 days after surgery. Univariate and multivariate analyses were performed by logistic regression. Internal validation of the model was performed using resampling techniques.
ResultsThe incidence of the outcome variable was 4.29% (95% CI 3.6–4.9). The variables remaining in the final logistic model were: age, sex, previous lung cancer resection, dyspnea (mMRC), right pneumonectomy, and ppo DLCO. The performance parameters of the model adjusted by resampling were: C-statistic 0.712 (95% CI 0.648–0.750), Brier score 0.042 and bootstrap shrinkage 0.854.
ConclusionsThe risk prediction model obtained from the GEVATS database is a simple, valid, and reliable model that is a useful tool for establishing the risk of a patient undergoing anatomic lung resection.
El objetivo es obtener un modelo predictor de riesgo quirúrgico en pacientes sometidos a resecciones pulmonares anatómicas a partir del registro del Grupo Español de Cirugía Torácica Video-Asistida (GEVATS).
MétodosSe recogen datos de 3.533 pacientes sometidos a resección pulmonar anatómica por cualquier diagnóstico entre el 20 de diciembre de 2016 y el 20 de marzo de 2018.
Definimos una variable resultado combinada: mortalidad o complicación Clavien-Dindo IV a 90 días tras intervención quirúrgica. Se realizó análisis univariable y multivariable por regresión logística. La validación interna del modelo se llevó a cabo por técnicas de remuestreo.
ResultadosLa incidencia de la variable resultado fue del 4,29% (IC 95% 3,6-4,9). Las variables que permanecen en el modelo logístico final fueron: edad, sexo, resección pulmonar oncológica previa, disnea (mMRC), neumonectomía derecha y DLCOppo. Los parámetros de rendimiento del modelo, ajustados por remuestreo, fueron: C-statistic 0,712 (IC 95% 0,648-0,750), Brier score 0,042 y Bootstrap shrinkage 0,854.
ConclusionesEl modelo predictivo de riesgo obtenido a partir de la base de datos GEVATS es un modelo sencillo, válido y fiable, y constituye una herramienta muy útil a la hora de establecer el riesgo de un paciente que se va a someter a una resección pulmonar anatómica.
Post-surgical complications impact significantly on hospital stay, postoperative mortality, and costs per patient.1
While many predictive models of surgical morbidity and mortality have been published for different types of intervention, in reality, none is used universally and routinely.
Thoracoscore, for example, was developed from a large French database for any type of chest surgery, including patients without lung cancer,2 and despite being validated in a sample of U.S. patients,3 it was insufficiently accurate when tested in other European patient groups.4,5 The French Society of Thoracic Surgery has recently published 2 surgical risk models for patients undergoing surgery exclusively for lung cancer based on a broader database than Thoracoscore (Epithor).6
For its part, the European Society of Thoracic Surgeons has developed its own predictive model (ESOS.01),7 which is calculated from 2 variables (FEV1 and age), but studies analyzing the accuracy of this model have drawn mixed conclusions.5,8,9 This European database for anatomical pulmonary resections was subsequently updated and the morbidity and mortality models revised.10 The situation with other types of predictive models is similar.11,12
A surgical risk model in thoracic surgery would help improve decision-making on the best therapeutic option for a given patient, and optimize the information we give to our patients.13,14
Models of this type can also be used as a quality of care indicator that allows researchers to compare outcomes from different thoracic surgery units. Moreover, objective knowledge of the surgical risk is of particular interest now that alternatives to surgery are being proposed for certain types of patients.
The aim of this study was to develop a surgical risk prediction model in patients undergoing anatomic lung resections using the database of the Spanish Video-Assisted Thoracic Surgery Group (GEVATS).15
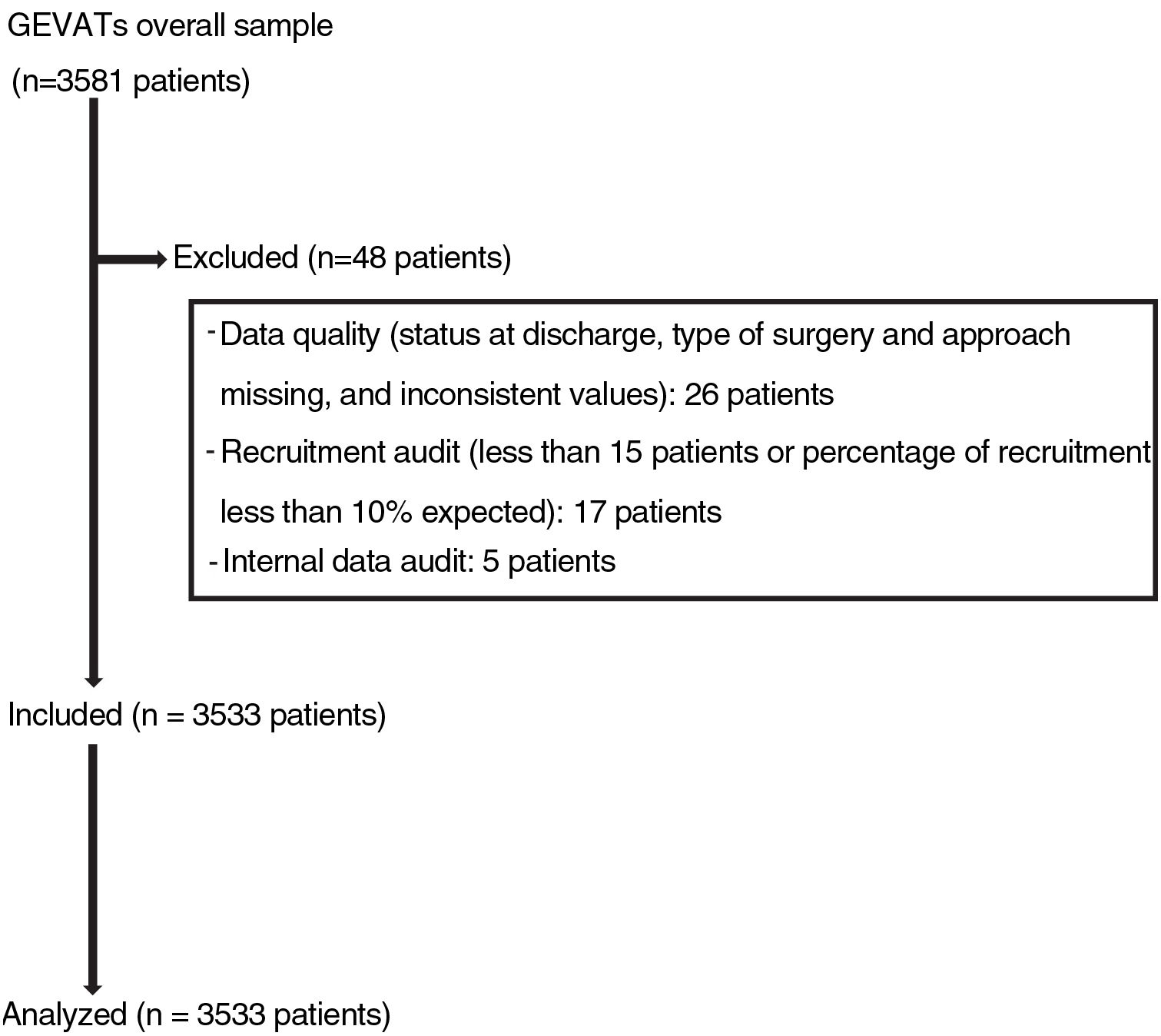
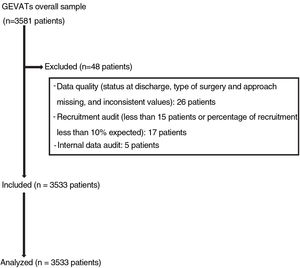
Materials and methodsIn 2015, the GEVATS database project was set up under the auspices of the Spanish Society of Thoracic Surgery, with the aim of analyzing the morbidity and mortality and oncological outcomes of lung resection surgery in Spain. An online database was developed that included patients undergoing anatomical pulmonary resection for any diagnosis and from any approach in all GEVATS thoracic surgery departments over a period of 15 consecutive months (December 20, 2016–March 20, 2018) (Fig. 1).
The project was approved by the Research Ethics Committee of all participating centers. All patients gave their informed consent for their clinical data to be used for scientific purposes.
Bilateral surgical procedures and patients under 18 years of age were excluded.
Demographic, functional, and comorbidity data were collected on the surgical procedure and on postoperative morbidity and mortality (at 90 days) (supplementary material).
All variables were defined according to the published guidelines of the Society of Thoracic Surgeons (STS) and the European Society of Thoracic Surgeons.16
Respiratory, cardiovascular and other complications were graded according to the Clavien-Dindo severity classification.17
Cases for whom no data were available on the type of pulmonary resection, type of surgical approach, and patient status at hospital discharge were excluded. Data from sites where recruitment was less than 10% of the expected rate or that recruited fewer than 15 patients during the study period were also excluded.
The quality of the data was ascertained by the members of the GEVATS scientific committee during an internal audit that consisted of comparing certain variables recorded in the database (date of surgery, type of resection, approach, hospital stay, grade IIIb-IV complications, and patient status at discharge) with the same variables recorded in the medical records of at least 20% of cases included by each of the participating centers.
Statistical analysisThe descriptive analysis was performed by obtaining absolute and relative frequencies for categorical variables, and mean and standard deviation or median and percentiles 25 and 75 for numerical variables.
The outcome variable was defined as a composite variable, in which the presence of any Clavien-Dindo grade IV complication within 90 days of the intervention or mortality from any cause was the event of interest. The univariate analysis was performed by testing the hypothesis using Pearson's Chi-squared test or Fisher's exact statistic for categorical variables and Mann–Whitney tests for numerical variables.
The variables that were significant in the univariate analysis and other clinically relevant variables (although not statistically significant) were used to develop the multivariate logistic predictive model. A collinearity diagnosis was performed between the independent variables included in the model, and those that met the criteria were eliminated. An automatic regression modeling strategy was applied, by which variables with a significance level in the Wald test of p>0.05 were successively removed from the model.
Internal validation of the model was performed using bootstrap resampling techniques with 100 replications, including measures of overall performance, calibration and discrimination. Calibration was evaluated using a calibration curve, discrimination using the C-statistic, and overall performance using the Brier score.18–21
Finally, to facilitate the interpretation of the prognostic models, a nomogram with the results of the final model was generated using the “nomolog” patch in STATA.22
The threshold for statistical significance was set at 0.05. The statistical analysis was carried out using the Stata/IC v.16 package (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
ResultsData on mortality and Clavien-Dindo grade IV complications from 3533 patients from 33 thoracic surgery departments were analyzed.
The characteristics of the sample and the variables analyzed are shown in Tables 1 and 2.
Other population variables.
| Variables | |
|---|---|
| Active smoker | 980 (28.37%) |
| Alcohol use | 224 (6.37%) |
| HBP | 1561 (44.33%) |
| Diabetes mellitus | 657 (28.66%) |
| Heart failure | 82 (2.32%) |
| Ischemic heart disease | 314 (8.92%) |
| Arrhythmia | 277 (7.87%) |
| Peripheral vascular disease | 318 (9%) |
| ACVA | 180 (5.1%) |
| mMRC dyspnea | |
| 0 | 2231 (63.45%) |
| 1 | 1000 (28.44%) |
| 2 | 252 (7.17%) |
| 3 | 33 (0.94%) |
| Liver failure | 33 (0.94%) |
| BMI | 26.86 (SD 4.57) |
| Albumin (g/dl) | 4.1(SD 0.54) |
| Creatinine (>2mg/dl) | 93 (2.64%) |
| Dementia | 19 (0.54%) |
| ppo FEV1 | 69.96%(SD 18.26) |
| ppo DLCO | 65.41% (SD 18.47) |
| ppo VO2max (ml/kg/min) | 13.83(SD 3.57) |
| ASA | |
| I | 81 (2.30%) |
| II | 1464 (41.69%) |
| III | 1876 (53.42%) |
| IV | 91 (2.59%) |
| Previous thoracic surgery (oncological) | 130 (4.23%) |
| Previous cardiac surgery | 63 (1.78%) |
| Surgeon's years of experience | |
| >20 years | 834 (23.69%) |
| 10–20 years | 1108 (31.45%) |
| <10 years | 1356 (44.92%) |
| House officer | 222 (6.31%) |
| Surgeon'sVATS experience | |
| >100 cases | 981 (27.87%) |
| 50–100 cases | 1015 (28.84%) |
| <50% cases | 1432 (40.68%) |
| No experience | 92 (2.61%) |
| Approach | |
| Open | 1616 (45.74%) |
| VATS | 1917 (54.46%) |
| Site | |
| Central | 1198 (39%) |
| Peripheral | 1873 (61%) |
| Lymph node involvement on imaging (CT) | |
| cN1 | 230 (7.5%) |
| cN2 | 315 (10.26%) |
| cN3 | 18 (0.59%) |
| Neoadjuvance | |
| CT | 245 (7.97%) |
| RT | 74 (2.41%) |
| Diagnosis | |
| Lung cancer | 3074 (87.33%) |
| Lung metastases | 244 (6.93%) |
| Other | 202 (5.74%) |
| Number of functioning segments resected | |
| 0 | 62 (1.76%) |
| 1–3 | 1632 (46.39%) |
| 4–5 | 1584 (45.03%) |
| ≥6 | 240 (6.82%) |
| Right pneumonectomy | 90 (2.55%) |
| Reintervention | 122 (3.46%) |
| Respiratory complications | 791 (22.39%) |
| Persistent air leak | 419 (11.86%) |
| Pneumonia | 163 (4.61%) |
| Atelectasis | 124 (3.51%) |
| Effusion | 78 (2.21%) |
| Reintubation | 53 (1.50%) |
| Empyema | 44 (1.25%) |
| ARDS | 44 (1.25%) |
| Bronchopleural fistula | 27 (0.76%) |
| PET | 13 (0.37%) |
| CD I | 312 (39.44%) |
| CD II | 208 (26.30%) |
| CD III | 199 (23.89%) |
| CD IV | 37 (4.68%) |
| CD V | 45 (5.69%) |
| Cardiovascular complications | 248 (7.02%) |
| Arrhythmias | 170 (4.81%) |
| ACVA | 5 (0.14%) |
| Heart failure | 20 (0.57%) |
| AMI | 3 (0.08%) |
| DVT | 1 (0.03%) |
| CD I | 33 (13.31%) |
| CD II | 170 (68.55%) |
| CD III | 12 (4.84%) |
| CD IV | 24 (9.68%) |
| CD V | 9 (3.63%) |
| Other complications | 236 (6.74%) |
| Gastrointestinal | 43 (1.22%) |
| Urologic | 82 (2.32%) |
| Psychiatric | 16 (0.45%) |
| CNS | 19 (0.54%) |
| Hematology | 18 (0.51%) |
| CD I | 97 (40.76%) |
| CD II | 98 (41.18%) |
| CD III | 17 (7.14%) |
| CD IV | 13 (5.46%) |
| CD V | 13 (5.46%) |
| Surgical wound infection | 50 (1.42%) |
| Survival at 90 days | 97.07% |
| Survival on discharge | 98.41% |
| Mean hospital stay | 5 days (P25-75: 4–7 days) |
| Readmission | 6.96% |
ACVA: acute cardiovascular accident; AMI: acute myocardial infarction; ASA: American Society of Anesthesiology Physical Status; CD: Clavien-Dindo classification (I–V); cN1: pathological hilar or intrapulmonary adenopathies on imaging; cN2: pathological ipsilateral mediastinal adenopathies on imaging; cN3: contralateral or ipsilateral supraclavicular mediastinal adenopathies on imaging; CNS: central nervous system; CT, chemotherapy; DVT: deep vein thrombosis; mMRC: modified Medical Research Council dyspnea scale; ppo FEV1: predicted postoperative FEV1. Calculated automatically in the database from the number of functioning segments resected; ppo DLCO: predicted postoperative DLCO. Automatically calculated in the database from the number of functioning segments resected; ppo VO2 max: predicted postoperative maximum O2 consumption. Automatically calculated in the database from the number of functioning segments resected; PTE: pulmonary thromboembolism; RT, radiation therapy; SDRA: adult respiratory distress syndrome; VATS: video-assisted thoracoscopic surgery.
The incidence of the composite variable (mortality at 90 days+grade IV complications at 90 days) was 4.29% (95% CI: 3.6–4.9).
There were 2 cases of intraoperative mortality (0.06%). Mortality at discharge was 1.59%, at 90 days it was 2.93%, and the rate of readmissions was 6.96%.
Median hospital stay was 5 days (P25 4 days; P75 7 days).
More than half of the patients in the sample underwent minimally invasive procedures (54.26%). The reconversion rate was 15.85%.
The most frequent postoperative complications were respiratory (22.39%), of which prolonged air leak was the most common (52.97%). The second most frequent type of complications were cardiovascular (7.02%), 68.51% of which were arrhythmias.
The incidence of more serious complications (Clavien-Dindo IV) in respiratory, cardiovascular and other organs and systems was 1.04%, 0.68% and 0.36%, respectively.
The variables associated with the composite outcome variable (mortality+Clavien-Dindo IV complication) are summarized in Table 3.
Univariate analysis.
| Variables | Outcome 90 daysNo. | Outcome 90 daysYes | p |
|---|---|---|---|
| Demographics | |||
| Sex | <0.001 | ||
| Female | 1036 (30.7%) | 20 (13.2%) | |
| Male | 2333 (69.25%) | 131 (86.65%) | |
| Age (years) | 64.77 (SD 10.11) | 68.14 (SD 8.40) | 0.001 |
| Comorbidities | |||
| Smoking | 0.001 | ||
| Never | 540 (16.3%) | 8 (5.4%) | |
| Former smoker≤12 months | 1383 (41.8%) | 76 (51.3%) | |
| Former smoker>12 months | 443 (13.4%) | 26 (17.6%) | |
| Smoker | 942 (28.5%) | 38 (25.7%) | |
| Unknown | 942 (28.5%) | 38 (25.7%) | |
| Alcohol | <0.001 | ||
| Yes | 204 (6.1%) | 20 (13.2%) | |
| No | 3165 (93.94%) | 131 (86.75%) | |
| Cardiovascular | 0.045 | ||
| HBP yes | 1482 (44.03%) | 79 (52.32%) | |
| HBP no | 1884 (55.97%) | 72 (47.62%) | |
| Diabetes mellitus | 0.096 | ||
| Yes | 621 (18.44%) | 36 (23.84%) | |
| No | 2747 (81.56%) | 115 (76.16%) | |
| Heart failure | 0.171 | ||
| Yes | 76 (2.26%) | 6 (3.97%) | |
| No | 3292 (97.74%) | 145 (96.03%) | |
| Ischemic heart disease | 0.013 | ||
| Yes | 292 (8.67%) | 22 (14.57%) | |
| No | 3077 (91.33%) | 129 (85.43%) | |
| Arrhythmias | 0.973 | ||
| Yes | 265 (7.87%) | 139 (92.05%) | |
| No | 3102 (92.13%) | 12 (7.95%) | |
| Peripheral vascular disease | 0.330 | ||
| Yes | 301 (8.94%) | 134 (88.74%) | |
| No | 3067 (91.06%) | 17 (11.26%) | |
| ACVA | 0.216 | ||
| Yes | 169 (5.02%) | 11 (7.28%) | |
| No | 3199 (94.98%) | 140 (92.72%) | |
| mMRC dyspnea grade | <0.001 | ||
| 0 | 2162 (64.25%) | 69 (45.70%) | |
| 1 | 940 (27.93%) | 60 (39.74%) | |
| 2 | 233 (6.92%) | 19 (12.58%) | |
| 3 | 30 (0.89%) | 3 (1.99%) | |
| Liver failure | <0.001 | ||
| Yes | 27 (0.80%) | 145 (96.03%) | |
| No | 3342 (99.20%) | 6 (3.97%) | |
| BMI | 26.85 (SD 4.58) | 26.96 (SD 4.39) | 0.783 |
| Albumin | 4.11 (SD 0.53) | 3.82 (0.71) | 0.032 |
| Creatinine (>2mg/dl) | 0.114 | ||
| Yes | 86 (2.55%) | 7 (4.67%) | |
| No | 3282 (97.45%) | 143 (95.33%) | |
| Dementia | 0.834 | ||
| Yes | 18 (0.53%) | 1 (0.66%) | |
| No | 3351 (99.47%) | 159 (99.34%) | |
| Functional parameters | |||
| ppo FEV1 | 70.40 (SD 18.18) | 59.97 (SD 17.20) | 0.001 |
| ppo DLCO | 65.85 (SD 18.36) | 56.18 (SD 16.50) | 0.001 |
| ppo VO2max | 13.97 (SD 3.54) | 12.34 (SD 3.65) | 0.001 |
| ASA | 0.002 | ||
| I | 81 (2.41%) | 0 | |
| II | 1417 (42.15%) | 47 (31.33%) | |
| III | 1781 (52.97%) | 95 (63.33%) | |
| IV | 83 (2.47%) | 8 (5.33%) | |
| Surgical procedure | |||
| Previous chest surgery (oncological) | 0.007 | ||
| Yes | 118 (4.02%) | 12 (8.76%) | |
| No | 2815 (95.98%) | 125 (91.24%) | |
| Previous cardiac surgery | 0.285 | ||
| Yes | 62 (1.84%) | 1 (0.66%) | |
| No | 3307 (98.16%) | 150 (99.34%) | |
| Surgeon's years of experience | 0.216 | ||
| >20 years | 806 (23.92%) | 28 (1.54%) | |
| 10–20 years | 1050 (31.17%) | 58 (38.41%) | |
| >10 years | 1299 (38.56%) | 57 (37.75%) | |
| House officer | 214 (6.35%) | 8 (5.30%) | |
| Surgeon'sVATS experience | 0.022 | ||
| >100 cases | 950 (28.20%) | 31 (20.53%) | |
| 50–100 cases | 962 (28.55%) | 53 (35.10%) | |
| >50 cases | 1373 (40.75%) | 59 (39.07%) | |
| No experience | 84 (2.49%) | 8 (5.30%) | |
| Site | 0.009 | ||
| Central | 1130 (38.51%) | 68 (49.64%) | |
| Peripheral | 1804 (61.49%) | 69 (50.36%) | |
| Lymph node involvement (CT) | 0.034 | ||
| cN0 | 2405 (82.03%) | 101 (73.72%) | |
| cN1 | 217 (7.40%) | 13 (9.49%) | |
| cN2 | 292 (9.96%) | 23 (16.79%) | |
| cN3 | 18 (0.61%) | 0 | |
| Neoadjuvant | 0.919 | ||
| Yes | 250 (8.51%) | 12 (8.76%) | |
| No | 2687 (91.49%) | 125 (91.24%) | |
| Diagnostic | 0.433 | ||
| Lung cancer | 2937 (87.18%) | 137 (90.73%) | |
| Lung metastases | 236 (7.01%) | 8 (5.30%) | |
| Others | 196 (5.82%) | 6 (3.97%) | |
| Functional segments resected | <0.001 | ||
| 0 | 56 (1.66%) | 6 (3.97%) | |
| 1–3 | 1568 (46.57%) | 64 (42.38%) | |
| 4–5 | 1529 (45.41%) | 55 (36.42%) | |
| ≥6 | 214 (6.36%) | 26 (17.22%) | |
| Right pneumonectomy | <0.001 | ||
| Yes | 71 (2.11%) | 19 (12.58%) | |
| No | 3298 (98.89%) | 132 (87.42%) | |
ACVA: acute cardiovascular accident; ASA: American Society of Anesthesiology Physical Status; BMI: body mass index; cN1: pathological hilar or intrapulmonary adenopathies on imaging; cN2: pathological ipsilateral mediastinal adenopathies on imaging; cN3: contralateral or ipsilateral supraclavicular mediastinal adenopathies on imaging; mMRC: modified Medical Research Council dyspnea scale; ppo DLCO: predicted postoperative DLCO, automatically calculated in the database from the number of functioning segments resected; ppo FEV1: predicted postoperative FEV1, calculated automatically in the database from the number of functioning segments resected; ppo VO2 max: predicted postoperative maximum O2 consumption, automatically calculated in the database from the number of functioning segments resected; VATS, video-assisted thoracoscopic surgery.
Albumin demonstrated a statistically significant association in the univariate analysis, but a high proportion of values were missing (53%). A missing-at-random pattern cannot be assumed, so this variable was eliminated from the definitive analysis.
Diabetes mellitus was added to the multivariate logistic regression analysis in addition to the variables that showed statistical significance, as this is associated with an increase in surgical morbidity and mortality in some series.23–25 Moderate-to-severe hepatic impairment and alcohol consumption showed a statistically strong association, but they were withdrawn from the final analysis because no specific alcohol consumption data or definition of the diagnosis or severity of liver failure were recorded in the database, preventing objective classification. The maximum model was built using age, sex, smoking habit, high blood pressure, ischemic heart disease, diabetes mellitus, previous lung cancer surgery, dyspnea grade, ASA score, tumor size, right pneumonectomy, FEV1 predicted, ppo DLCO and surgeon's experience with VATS. After backward elimination, the variables age, sex, previous lung cancer surgery, dyspnea, right pneumonectomy, and ppo DLCOppo remained in the final model (Table 4).
Multivariate analysis.
| OR (95% CI) | p-Value | |
|---|---|---|
| Age | 1.03 (1.00–1.05) | 0.007 |
| Sex (Female) | 0.30 (0.16–0.57) | <0.001 |
| Previous lung cancer | 2.02 (1.00–4.10) | 0.049 |
| Dyspnea (mMRC) | 1.87 (1.26–2.77) | 0.002 |
| Right pneumonectomy | 4.06 (2.05–8.03) | <0.001 |
| ppo DLCO | 0.98 (0.01–0.39) | 0.004 |
mMRC: modified Medical Research Council dyspnea scale; ppo DLCO: predicted postoperative DLCO, automatically calculated in the database from the number of functioning segments resected.
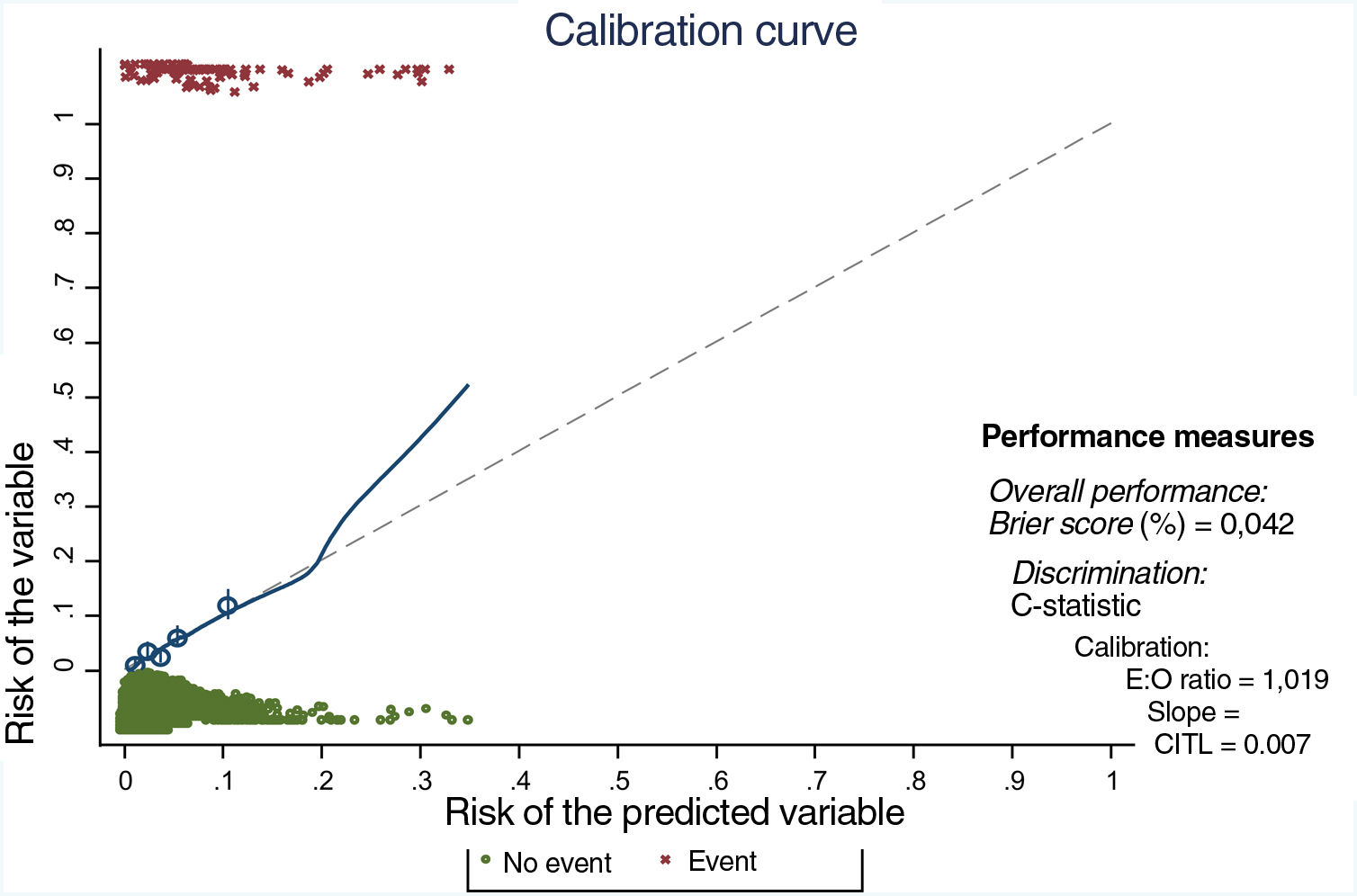
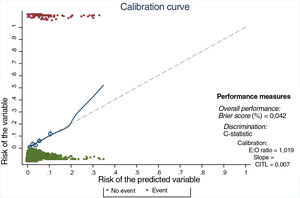
One hundred subsamples were obtained by bootstrap resampling techniques, and the modeling strategy was repeated for each one. The performance parameters of the model, adjusted by bootstrapping, are shown in Table 5, and show good outcomes in performance, calibration (Fig. 2) and discrimination.
Calibration curve. E:O ratio: ratio between the number of estimated cases and the number of observed cases; CITL: calibration in the large. The ideal calibration is shown in the curve by the dotted line and the match between expected and observed risk on the solid line. The line fits well in most quintiles (shown by circles), and only deviates when there are a small number of observations (after a predicted risk of 20%).
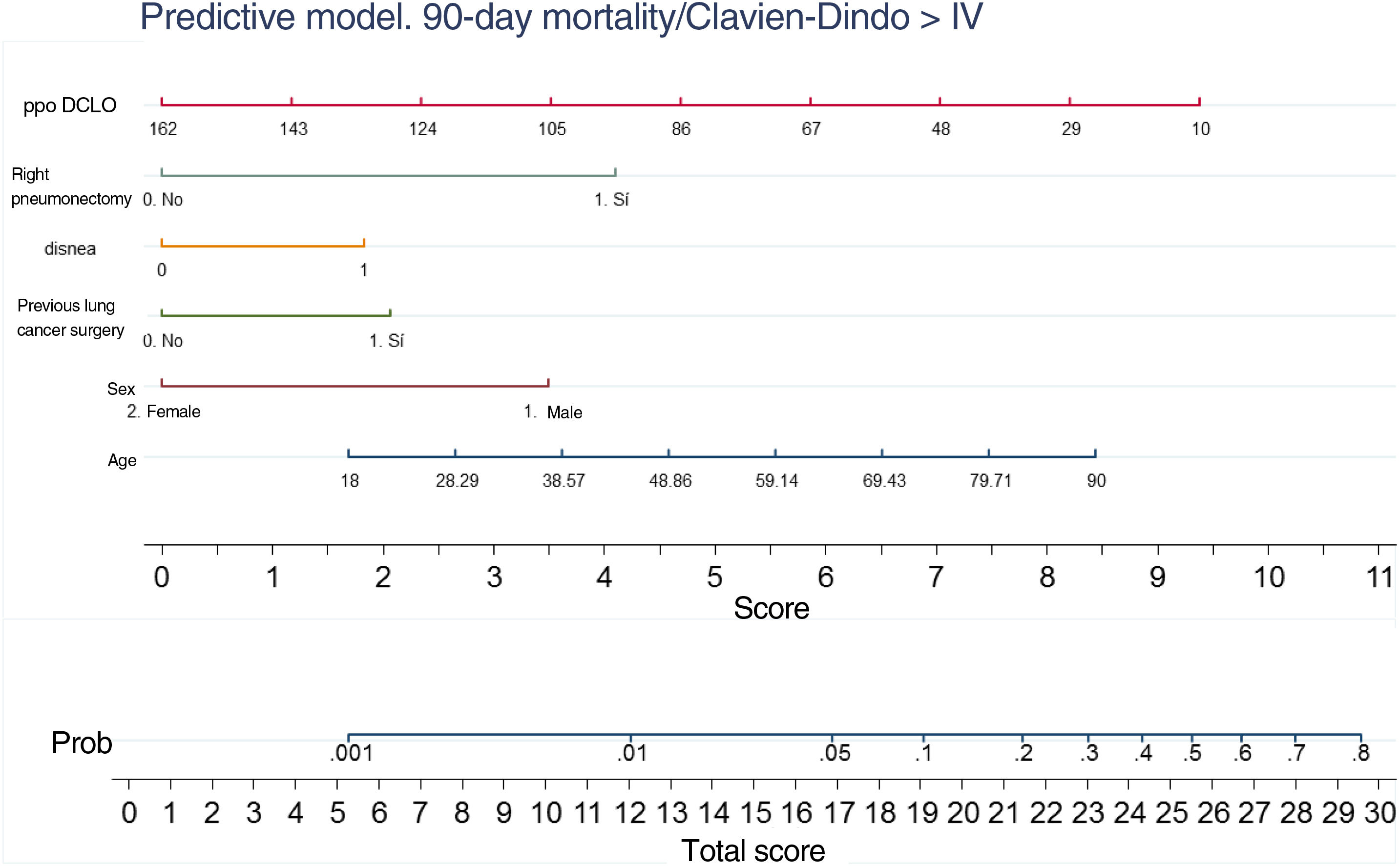
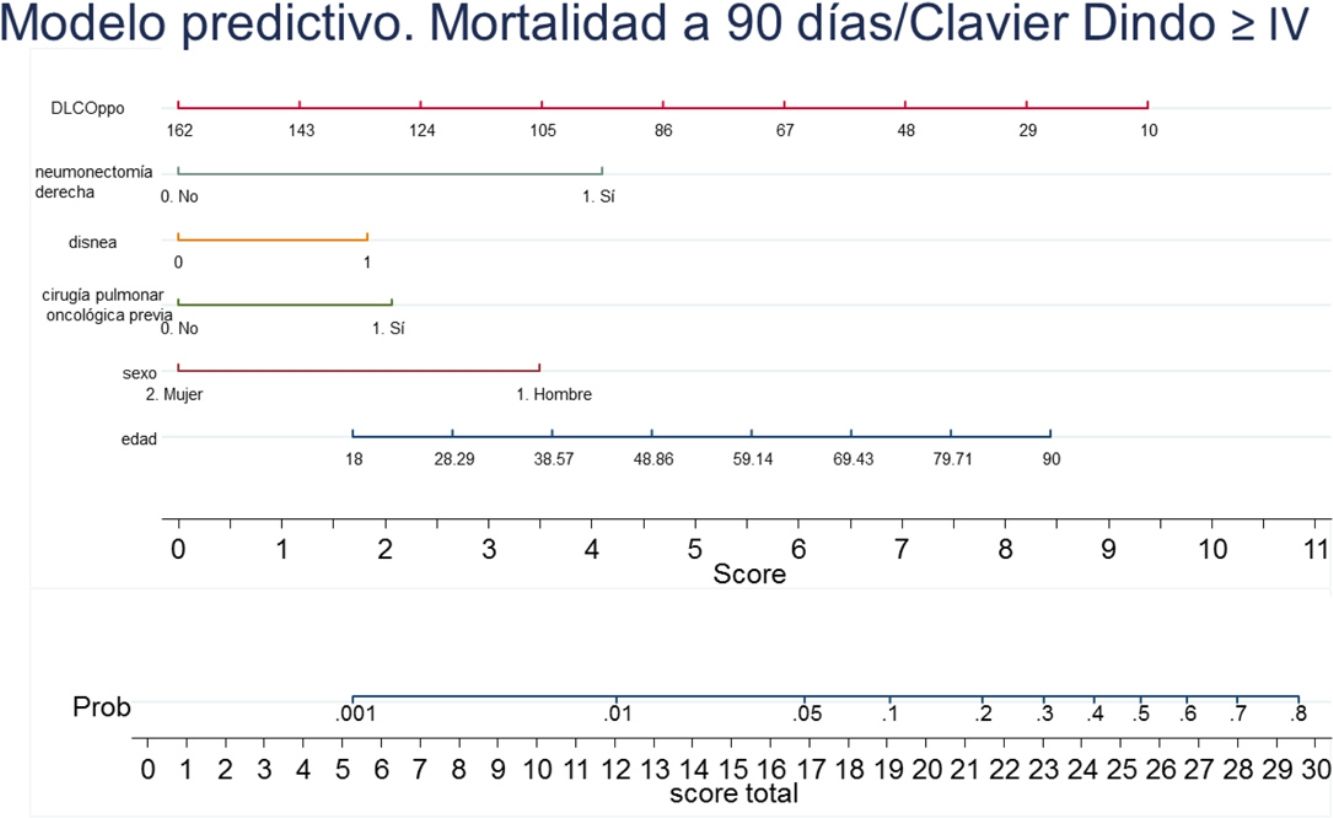
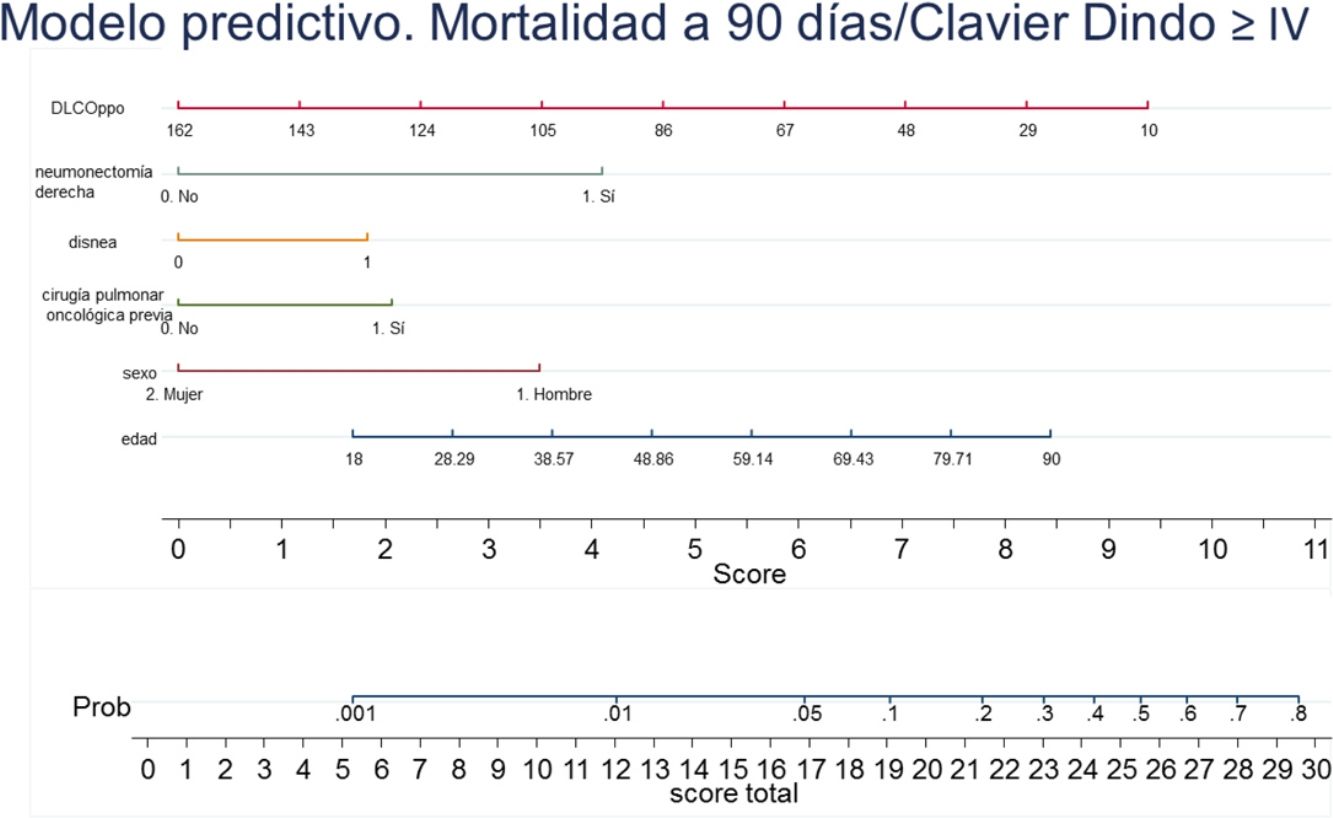
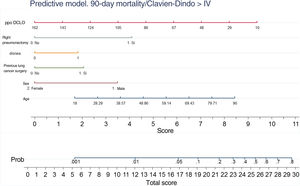
A nomogram was generated using the results of the final logistic regression analysis to graphically show the weight of each variable. The total score, obtained by adding the points corresponding to each of the variables in the final model, represents the predicted probability of experiencing an event, death or Clavien-Dindo≥IV complication at 90 days (Fig. 3). For example, a male patient (3.5 points), 78 years of age (7 points), with dyspnea on moderate exertion (2 points), who underwent lung resection for previous adenocarcinoma (2 points), who has a ppo DLCO of 50% (6.5 points), who is a candidate for anatomical resection will have a total score of 22, corresponding to a 20% risk of 90-day postoperative mortality or major complications. The risk in the same patient, but with DLCO 100%, falls to 10%, and to 5% if it is his first lung resection.
DiscussionA tool to anticipate which patients may develop significant complications after surgery is essential for making decisions about the best treatment available in each situation, and for comparing the performances of the different units providing a service – in this case, anatomical lung resections.
The morbidity and mortality outcomes in our series are comparable to those of other large published series.2,10,12 However, while the evidence suggests that approximately 15% of patients requiring anatomical resection undergo minimally invasive surgery, it is interesting to note that in our series the percentage of patients undergoing video-assisted thoracoscopic surgery was significantly higher (54.26%). This may be explained by the more rapid implementation of minimally invasive techniques in Spain than in neighboring countries, and possibly by the high percentage of procedures performed by thoracic surgery specialists.
Certain functional parameters and comorbidities are known to be associated with an increase in perioperative risk in patients undergoing lung resections, and various predictive models of morbidity and mortality have been designed, mainly based on national databases.1,2,10,11 However, none of these models is used universally.
This low uptake may be due to several reasons. Sometimes, genetic characteristics and health and social welfare conditions that are applicable in one population are not applicable in another. For example, Thoracoscore, a surgical risk model developed from a French database, does not perform well in the U.S. population. Furthermore, many of these models are complex to apply on a day-to-day basis, and use variables that are not available during consultation with the patient to inform them of their options (extended resections, type of approach, and disease staging).
Our aim with this model is to predict the surgical risk of a patient using the variables immediately available during the visit. It is not uncommon, given preoperative findings or even the judgment of the surgeon, for the complexity of the proposed intervention to be overestimated. In addition, interventions that are not necessarily technically difficult (e.g., intrapericardial pneumonectomy or partial resection of the diaphragm, chest wall, pericardium, etc.) are classed as extended surgeries in many publications.10,12
Age and sex are variables that are persistently associated with increased surgical risk. Similarly, the dyspnea grade indicates a patient's cardiorespiratory status and has been shown in many studies to have a prognostic impact in patients with respiratory disease.2,10,12
Among the current recommendations for a comprehensive study on the operability of a patient undergoing lung anatomical resection, predicted postoperative DLCO (ppo DLCO) is accepted as the most reliable indicator of the patient's functional reserve, to a greater extent even than FEV1.26 However, the models available to date do not include ppo DLCO, mainly because it is not widely performed. We did not include FEV1 as a predictor in our model, but we did include ppo DLCO, in line with the available scientific evidence.
Right pneumonectomy consistently appears as a negative prognostic factor in published series,27 and our analysis confirmed its impact on the morbidity and mortality of patients.
It is interesting to note the predictive value of a previous history of lung cancer. This variable is not included in any of the models described so far, except for the latest revision of Ephitor.6 It is estimated that about 6% of patients who undergo lung cancer resection will develop a second primary tumor, and now, thanks to screening CT scans, it is now increasingly common to detect resectable lesions in the earliest stages even on more than 1 occasion, permitting a more conservative approach,. Establishing the added risk of a second intervention in a given patient can often guide the decision between a surgical approach or other therapeutic options.
The reliability parameters of our model are acceptable, especially for low scores. The model tends to overestimate higher risk scores, probably because the number of events is small compared to the number of predictor variables included in the model.
This study has some limitations:
- -
First of all, it is based on a voluntary database. This may result in significant selection bias. However, it should be noted that patients from sites with low recruitment rates were excluded (median overall recruitment was 99% [p25–p75: 76–100%]), and an internal audit of data quality was carried out, revealing a degree of concordance of 98%.15
- -
Blood albumin levels, which in several studies have shown a prognostic impact on patients undergoing surgery, could not be analyzed because of the high percentage of patients for whom these data were missing. Other variables that might be of interest in assessing a patient's surgical risk, such as advanced liver disease or alcohol consumption, were collected in the database, but the lack of a clear definition meant that they had to be excluded from the analysis.
- -
In our analysis, we included all patients undergoing anatomical lung resection, regardless of their diagnosis. Most series include only patients with a lung cancer diagnosis without considering the type of lung resection performed.6,12 The diagnosis obviously has prognostic implications for the patient, but it seems logical to believe that complications in the immediate postoperative period will be more closely related to the type of resection performed and to the patient's current clinical situation (operability). The gold standard in the surgical treatment of lung cancer is lobectomy, and in some very specific cases, anatomical sublobar resection. In our opinion, the GEVATS series is more representative of patients who are candidates for surgical treatment with curative intent for any diagnosis than other larger series.
We conclude that the predictive risk model obtained from this database is a simple, reliable model that constitutes a highly useful tool in classifying patients undergoing anatomical lung resection, and one that helps empower patients when making decisions about their treatment.
FundingThe costs of setting up and maintaining the GEVATS database were covered by Ethicon, Johnson & Johnson. The authors have had absolute freedom and control in all aspects of the design, methodology, analysis and writing of the study.
GEVATS received a research grant from the Spanish Society of Thoracic Surgery in 2015.
Conflict of interestsThe authors state that they have no conflicts of interest.
We thank Johnson & Johnson for their collaboration in the development of the GEVATS database. We also thank all the clinical documentation staff at the participating centers for their collaboration in the audit.