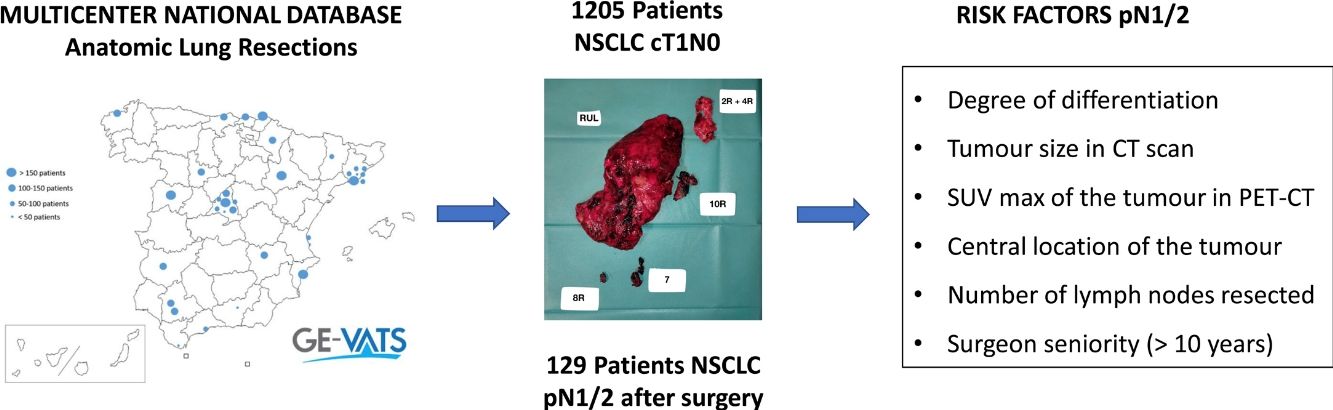
To determine the incidence of occult N1/N2 nodal metastases and associated risk factors in patients with non-small cell lung cancer no larger than 3cm and deemed cN0 by CT and PET-CT in a prospective, multicentre national database.
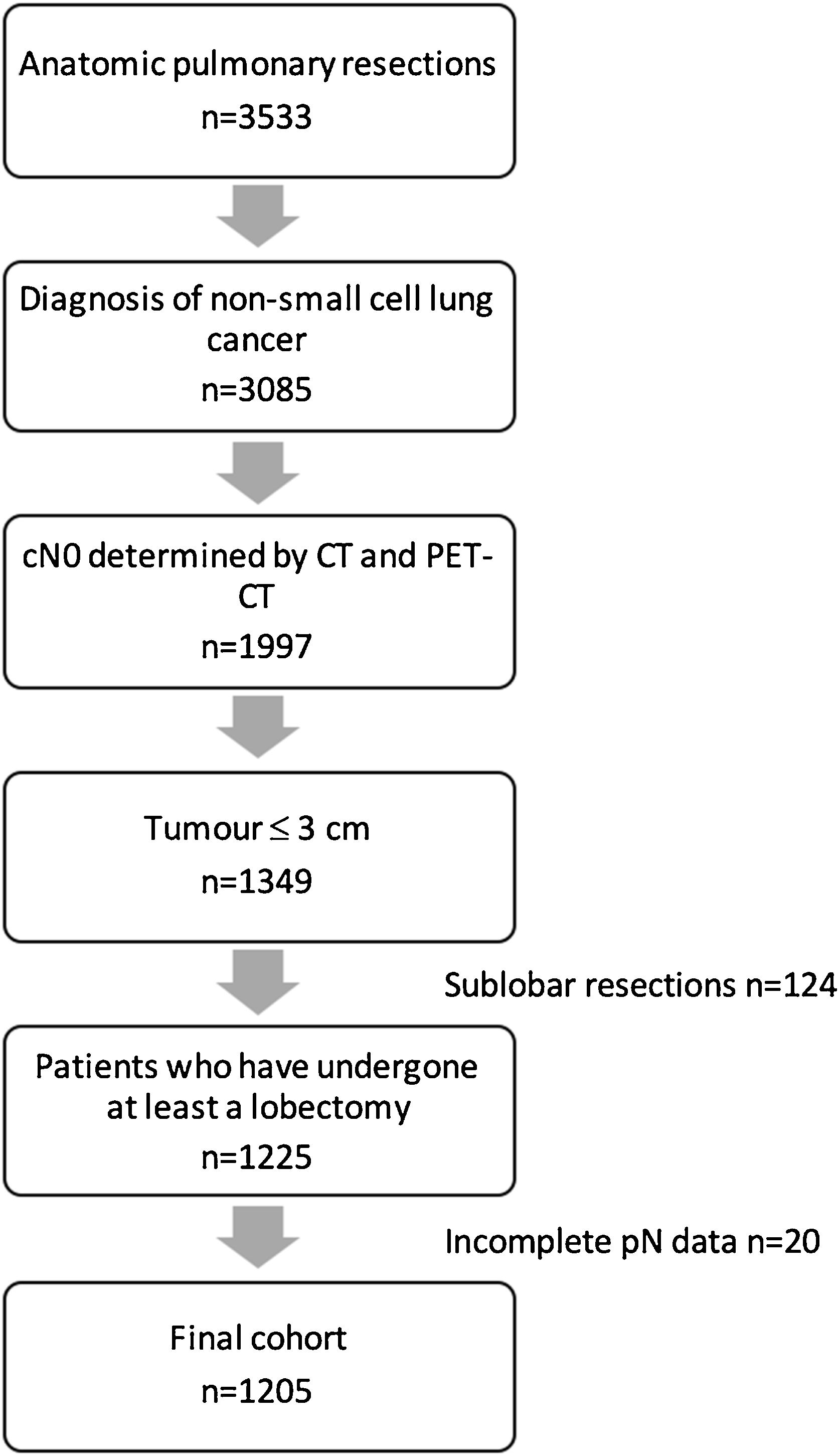
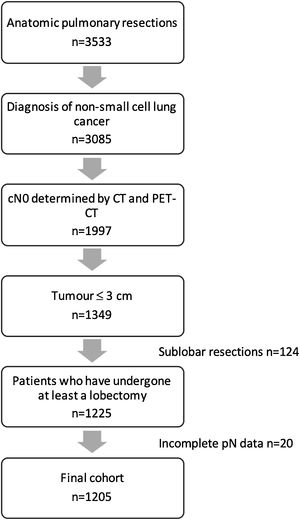
MethodsPatients with a NSCLC no larger than 3cm, deemed cN0 by PET-CT and CT scan, who had undergone at least a lobectomy, were selected from a national multicentre database of 3533 patients who had undergone anatomic lung resection between 2016 and 2018.
Clinical and pathological variables of patients with pN0 and patients with pN1/N2 were compared to identify factors associated with the presence of lymph node metastases. Chi2 and the Mann–Whitney U test were used for categorical and numerical variables, respectively. All variables with p<0.2 in the univariate analysis were included in the multivariate logistic regression analysis.
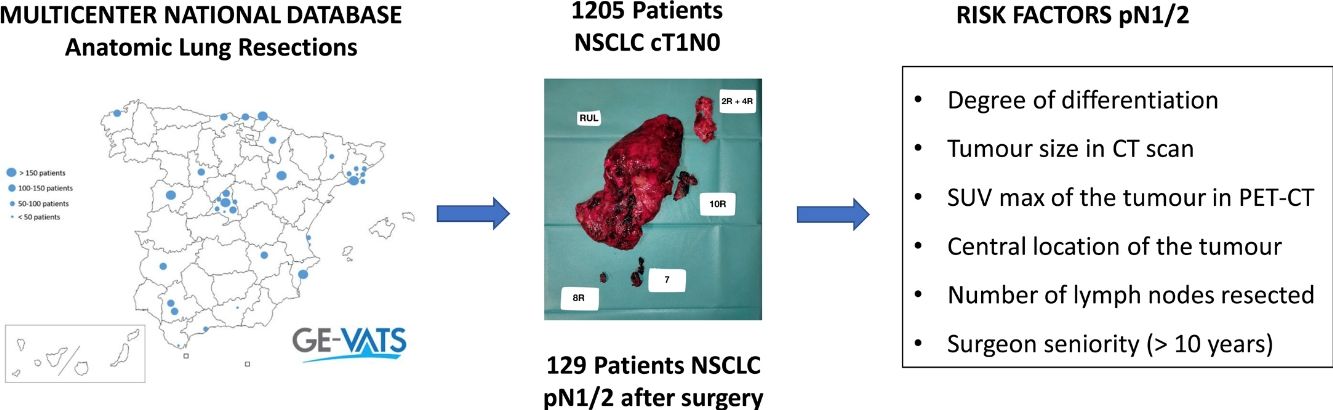
ResultsThe study included 1205 patients from the cohort. The incidence of occult pN1/N2 disease was 10.70% (95%CI, 9.01–12.58).
The multivariable analysis revealed that the degree of differentiation, size, location (central or peripheral) and SUV of the tumour in PET, surgeon experience and number of lymph nodes resected were associated with occult N1/N2 metastases.
ConclusionsThe incidence of occult N1/N2 in patients with bronchogenic carcinoma with cN0 tumours no larger than 3cm is no negligible. Data about the degree of differentiation, tumour size in CT scan, maximal uptake of the tumour in PET-CT, location (central or peripheral), number of lymph nodes resected and surgeon seniority is relevant in order to detect patients at risk.
Surgery remains the only curative option for operable patients with non-small cell lung cancer (NSCLC). In recent years, the paradigm of surgical treatment for early-stage patients has been changing, with anatomical sublobar resection being considered in selected cases.1 In inoperable patients or those who refuse surgery, non-surgical treatment such as stereotactic ablative radiotherapy (SABR) is an alternative treatment option. SABR is a strategy that employs very high doses of radiation delivered to the cancer target in a limited number of treatment fractions; this technique has demonstrated rates of local control over 90%, comparable to surgery.1 The encouraging results in this population have led to an increased interest in comparing the results of surgery and SABR in operable patients. Some randomised controlled trials have been attempted in the past, but were terminated early on due to poor recruitment.2
A relevant disadvantage of SABR is the lack of final pathologic analysis and lymph node assessment. Despite improvements in clinical staging with the implementation of CT and PET-CT, unexpected nodal metastases have been reported in up to 20% of patients with early-stage NSCLC. Therefore, the absence of pathologic staging could lead to understaging and prevent patients from receiving adjuvant therapies, resulting in a negative impact on survival.
For this reason, knowing the risk that patients with early-stage bronchogenic carcinoma have of developing intrapulmonary, hilar or mediastinal lymph node metastases is significant. The aim of the study is to determine the incidence of occult N1/N2 in patients with clinical stage IA NSCLC in a multicentre, prospective national database, and to analyse the factors associated with occult N1/N2 disease in this dataset.
Material and methodsEthics statementThe project was approved by the Ethics and Clinical Research Committees of each participating centre. All patients signed a specific informed consent for the use of their clinical data for scientific purposes.
Data sourceIn May 2015, the Spanish Society of Thoracic Surgeons (SECT) founded the GEVATS project, a multicentre, prospective national database to record all anatomic pulmonary resections performed in our country within a 15-month period (from 20 December 2016 to 20 March 2018), with the aim of analysing the implementation of minimal invasive procedures.
Variables regarding demographics, staging and pathological diagnosis, surgical procedure, morbimortality, and oncological follow up were recorded. Detailed data on the variable definitions are described elswhere.3 Tumours located in the 1/3 inner part of the lung were considered to have a central location. Data about lymphadenectomy included regions explored at the surgical procedure and number of lymph nodes resected per region in the final pathological analysis. Staging was classified according to the eighth edition of the American Joint Committee on Cancer. Patients who had undergone bilateral procedures and were under the age of 18 were excluded. An exhaustive audit of the database was performed3 and records were excluded if: (1) Key variables were missing; (2) The data was taken from centres with recruitment rates lower than 10%; (3) The inclusion criteria were not respected. Finally, the database included 3533 anatomical pulmonary resections from 33 Spanish thoracic surgery departments.
Study designPatients with a non-small cell lung cancer (NSCLC) no larger than 3cm, deemed cN0 by CT (<1cm in the short-axis diameter) and PET (after evaluation by nuclear medicine specialists), who had undergone at least a lobectomy, were selected from the database (Fig. 1). Clinical and pathological variables of patients with pN0 status were compared with those with pathological positive lymph nodes (pN1/N2).
The Chi2 test was used for comparing categorical variables and the Mann–Whitney U test for numerical variables. All variables with p<0.2 in the univariable analysis were included in the multivariable logistic regression analysis. An automatic backward strategy was performed and those variables with a p value<0.05 were retained in the final model. Statistical analyses were performed with Stata v17 (Stata Corp 2021. Stata Statistical Software: Release17. TX: StataCorp LLC).
ResultsThere were 1205 (34.1%) patients in the entire cohort who had undergone at least a lobectomy for NSCLC clinical stage cT1, deemed cN0 by CT scan and PET-CT, and were included in the analysis. Of these, there were 129 (10.7%,) patients in whom occult lymph node metastases was found in the final pathological analysis. Pathological N1 was identified in 73 patients (6.1%) and occult pN2 involvement in 56 (4.6%).
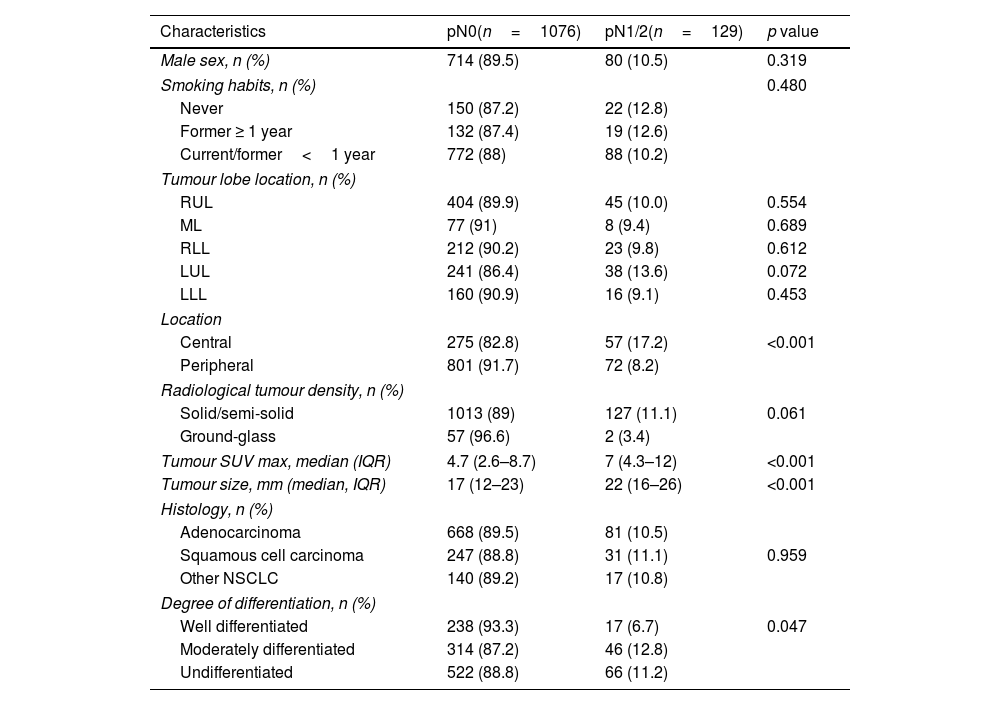
A comparison of clinical and pathologic characteristics can be found in Table 1.
Clinical and pathological characteristics (n=1205).
| Characteristics | pN0(n=1076) | pN1/2(n=129) | p value |
|---|---|---|---|
| Male sex, n (%) | 714 (89.5) | 80 (10.5) | 0.319 |
| Smoking habits, n (%) | 0.480 | ||
| Never | 150 (87.2) | 22 (12.8) | |
| Former ≥ 1 year | 132 (87.4) | 19 (12.6) | |
| Current/former<1 year | 772 (88) | 88 (10.2) | |
| Tumour lobe location, n (%) | |||
| RUL | 404 (89.9) | 45 (10.0) | 0.554 |
| ML | 77 (91) | 8 (9.4) | 0.689 |
| RLL | 212 (90.2) | 23 (9.8) | 0.612 |
| LUL | 241 (86.4) | 38 (13.6) | 0.072 |
| LLL | 160 (90.9) | 16 (9.1) | 0.453 |
| Location | |||
| Central | 275 (82.8) | 57 (17.2) | <0.001 |
| Peripheral | 801 (91.7) | 72 (8.2) | |
| Radiological tumour density, n (%) | |||
| Solid/semi-solid | 1013 (89) | 127 (11.1) | 0.061 |
| Ground-glass | 57 (96.6) | 2 (3.4) | |
| Tumour SUV max, median (IQR) | 4.7 (2.6–8.7) | 7 (4.3–12) | <0.001 |
| Tumour size, mm (median, IQR) | 17 (12–23) | 22 (16–26) | <0.001 |
| Histology, n (%) | |||
| Adenocarcinoma | 668 (89.5) | 81 (10.5) | |
| Squamous cell carcinoma | 247 (88.8) | 31 (11.1) | 0.959 |
| Other NSCLC | 140 (89.2) | 17 (10.8) | |
| Degree of differentiation, n (%) | |||
| Well differentiated | 238 (93.3) | 17 (6.7) | 0.047 |
| Moderately differentiated | 314 (87.2) | 46 (12.8) | |
| Undifferentiated | 522 (88.8) | 66 (11.2) | |
Number of patients; pN: pathologic nodal metastasis; SUV max: maximal uptake in PET; RUL: right upper lobe; ML: middle lobe; RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe; NSCLC: non-small cell lung cancer.
No differences were found as regards age, sex or smoking habits. The incidence of pathologic lymph node metastasis (pN+) was similar regardless lobe location, although significant differences were found when exploring the centrality of the tumour; out of the 332 centrally located tumours, 57 (17.2%) had pathological nodal involvement compared to 72 (8.2%) of the 873 peripheral tumours (p<0.001). Patients with nodal upstaging had larger tumours (medians of 22cm and 17cm respectively) and higher maximum uptake in PET CT (a median of 7 compared to 4.7), with the difference being statistically significant (p<0.001).
The pathological analysis showed that adenocarcinoma was the most frequent histology in both groups. Lymph node metastases findings were similar among the different histological subtypes, although differences were found when assessing tumour degree of differentiation; only 17 (6.7%) well-differentiated tumours had nodal metastases, while nodal metastases were found in 46 (12.8%) and 66 (11.2%) moderately differentiated and undifferentiated tumours, respectively (p=0.047).
The number of lymph nodes resected at surgery also showed association, being slightly higher in patients with occult nodal disease. The median number of nodes resected in the pN+group was 7 (IQR 5–12) compared to 6 (IQR 4–10) in the pN0 cohort (p=0.023).
As regards the surgical approach, a minimally invasive procedure was performed in 839 patients (69.6%) compared to 366 patients (30.4%) in whom the final approach was thoracotomy with rib spreading, with lymph node metastases being found in 70 (8.34%) and 59 patients (16.21%), respectively, with a statistically significant difference (p<0.001).
Regarding surgeon experience, more than 10 years of expertise was statistically related to pN+, whereas experience in minimally invasive approaches was not. Nodal metastases were present in 50 patients (8.62%) of the cohort operated on by junior thoracic surgeons (<10 years’ experience) compared to 79 (12.64%) of patients operated by senior thoracic surgeons (>10 years’ experience), p=0.024.
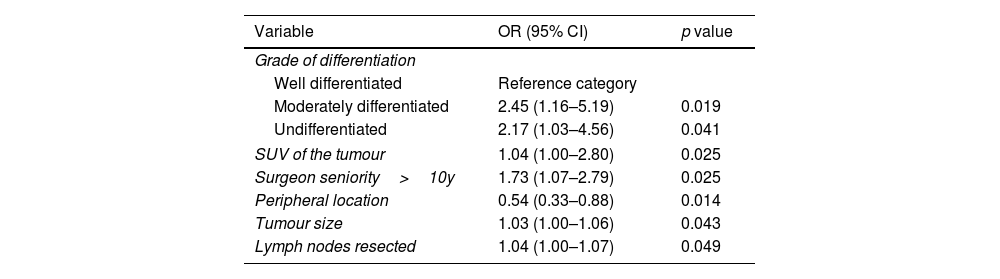
The multivariable analysis revealed that the degree of differentiation (either moderate or undifferentiated), tumour size in CT scan, maximal uptake of the tumour in PET-CT, location (central or peripheral), number of lymph nodes resected and surgeon seniority (>10y) were independent risk factors for occult nodal metastases (Table 2).
Multivariable analysis.
| Variable | OR (95% CI) | p value |
|---|---|---|
| Grade of differentiation | ||
| Well differentiated | Reference category | |
| Moderately differentiated | 2.45 (1.16–5.19) | 0.019 |
| Undifferentiated | 2.17 (1.03–4.56) | 0.041 |
| SUV of the tumour | 1.04 (1.00–2.80) | 0.025 |
| Surgeon seniority>10y | 1.73 (1.07–2.79) | 0.025 |
| Peripheral location | 0.54 (0.33–0.88) | 0.014 |
| Tumour size | 1.03 (1.00–1.06) | 0.043 |
| Lymph nodes resected | 1.04 (1.00–1.07) | 0.049 |
SUV max: maximal uptake in PET.
Clinical stage I NSCLC 5-year survival rates range from 77% to 92%, with nodal metastases being the main prognostic factor in this group of patients.4 Life expectancy drastically decreases to 56% and 41% when pathological N1 or N2, respectively, are found after surgery. In this scenario, adjuvant chemotherapy has shown to improve 5-year overall survival by 5%.5,6 The role of immunotherapy is less defined in resected patients, although encouraging results obtained in the neoadjuvant setting have led to multiple studies exploring the role of targeted therapy in the adjuvant setting, in this regard, pathological analysis of specimens and the molecular profiles of tumours are essential to guide treatment.
Despite improvements in radiological preoperative assessments of lung cancer, the combination of CT and PET-CT is not reliable enough to identify micrometastasis in early stages. In this analysis of a prospective, multicentre cohort of Spanish patients, 10.7% of patients with NSCLC≤3cm, deemed cN0 by CT and PET-CT, were upstaged to pN1 or pN2 disease after lobectomy and lymph node dissection, with degree of differentiation, tumour size, maximal uptake of the tumour in PET-CT, location (central or peripheral), number of lymph nodes resected and surgeon seniority being independent risk factors in the multivariable analysis. The paramount relevance of this issue has led to multiple studies exploring the risk factors of unpredicted nodal metastases in patients with presumed early stage NSCLC, although results differ from one to another, all of our findings have been previously mentioned in literature.
Tumour size is a relevant prognostic factor in lung cancer, even in the early stages, and it has been reported as a risk factor for occult nodal disease in some publications, although the cut-off point varies from one to another. Chen et al. found that 13.8% of patients with tumours ≤1cm had nodal metastases, compared to 31.6% in patients with tumours measuring 2–3cm.7 Zhao et al.8 determined a cut-off point greater than 2.65cm as a risk factor; for Fang et al.,9 tumours greater than 1.7cm in size had an increased risk for occult nodal disease, whereas in the publication by Zhang et al., patients with tumours larger than 1cm were at risk.10
The predictive value of various biomarkers has been explored in multiple publications. A meta-analysis published by Nasralla et al. exploring the role of serum biomarkers in the prognosis of lung cancer found that CEA level is related to nodal involvement and survival of lung cancer patients.11 Several studies, most of which conducted in Asia, confirm this hypothesis, although the cut-off value still needs to be set.7,9,10,12–14 We were not able to analyse the potential impact of biomarkers in our cohort because they were not included in the design of the GEVATS database.
The results among different studies vary concerning tumour histology,7,10,12,15,16 but tumour biology consistently seems to be an important risk factor for lymph node involvement. Most of our patients with unsuspected nodal disease had moderately differentiated (12.8%) or undifferentiated (11.2%) tumours compared to 6.7% in the group of well differentiated tumours. This finding is in line with a multicentre study conducted in China7 wherein only 2% of well-differentiated tumours developed nodal metastases compared to 13.6% and 36.2% of moderately and poorly differentiated tumours, respectively.
Location of the tumour is another key issue with concerning the presence of occult lymph node disease. Centrally located tumours are considered a risk factor for mediastinal lymph node involvement, and within our cohort they are the only tumours that have an indication for invasive staging according to the current ESTS guidelines.17 However, there is controversy in the literature in this regard; firstly, due to the lack of consensus on the definition of “central tumour” and, secondly, because several publications focused on the review of central tumours found a relationship with the presence of N1 metastases, but not N2.18,19 The relationship of central tumours with occult N1 involvement is irrelevant in the case of patients who undergo lobectomy, but it is crucial when considering sublobar resections or non-surgical treatments.
Lymphadenectomy quality and precision parameters have not been included in our analysis. The minimum standard recommended by the International Association for the Study of Lung Cancer (IASLC) is at least three mediastinal lymph nodes (always including region 7) and three hilar and/or intralobar nodes.20 According to Obiols et al., only 65% of lung cancer patients in the GEVATS database met the minimum criteria proposed by the IASLC, with a median of seven lymph nodes sampled.21 We found that upstaged patients had a median of seven nodes resected compared to six in the pN0 cohort, and the differences was statistically different among groups. Therefore, non-compliance with the IASLC minimum criterion may result in an underestimation of nodal upstaging, although the analysis reflects the real assessment and performance of lymphadenectomy in our country.
According to current guidelines, invasive mediastinal staging can be omitted in stage-IA lung cancer patients because of low pre-test probability for N2 disease in this context,17 although its role in patients who are not candidates for surgery is not defined. Some studies have explored the ability of invasive staging techniques to identify occult mediastinal metastases in stage-IA lung cancer. Resio et al. explored this issue in a cohort of 30685 stage-I lung cancer patients from the Society of Thoracic Surgeons database; they found that 19% of patients with negative invasive staging had lymph node metastases upon pathological analysis.22
Nevertheless, we must consider to perform non-invasive staging techniques in patients with risk factors of occult nodal disease, especially if we consider non-surgical techniques.
The aim of this study is to reinforce the argument that occult nodal metastases is not infrequent in early-stage non-small cell lung cancer, and therefore, it is essential surgical resection combined with an appropriate lymphadenectomy be offered to every patient who tolerates and accepts surgical resection in order to obtain the best curative treatment.
Our study has several limitations. First of all, the database was not specifically designed for the purpose of the study, therefore some variables potentially associated with occult metastases such as CEA level, consolidation-to-tumour ratio or tumour spread through air spaces (STAS) were not included. Secondly, its multicentre nature could have led to differences among centres concerning preoperative staging, extent of lymphadenectomy, or pathological processing of specimens. However, the prospective nature of the database, the quality of recruitment and previously audited data,3 and the fact that it represents approximately 50% of national thoracic surgical activity in said time period, lend a high degree of reliability and representativeness to our results.
ConclusionThe incidence of occult N1/N2 in patients with early-stage bronchogenic carcinoma is not negligible, data about the degree of differentiation, tumour size in CT scan, maximal uptake of the tumour in PET-CT, location (central or peripheral), number of lymph nodes resected and surgeon seniority is relevant in order to detect patients at risk.
Author contribution statementAlejandra Romero Román: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing.
Silvana Crowley Carrasco: Writing—review & editing.
Mariana Gil Barturen: Writing—review & editing.
Ana Royuela: Data curation; Formal analysis; Methodology; Writing—review & editing.
Carme Obiols: Writing—review & editing.
Sergi Call: Writing—review & editing.
José Luis Recuero: Writing—review & editing.
Íñigo Royo: Writing—review & editing.
Raúl Embún: Data curation; Writing—review & editing.
David Gómez de Antonio: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing.
The GEVATS members: Data collection.
Data availability statementThe data underlying this article will be shared on reasonable request to the corresponding author.
Funding statementAll costs related to the start-up and maintenance of the GEVATS database were covered by Ethicon, Johnson & Johnson. The authors had freedom of investigation and full control of the design of the study, methods used, outcome parameters and results, data analysis, and production of the written report. The GEVATS was awarded a grant from the Spanish Society of Thoracic Surgery for the best national research project of 2015.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Johnson & Johnson for their collaboration in the development of the Spanish VATS group. We also thank all those responsible for the clinical documentation services of each hospital for actively participating in the audit of the dataset. The GEVATS members are as follows: Sergio Bolufer (Servicio de Cirugía Torácica, Hospital General Universitario de Alicante, Alicante), Miguel Congregado (Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla), Marcelo F. Jiménez (Servicio de Cirugía Torácica, Hospital Universitario de Salamanca, Universidad de Salamanca, IBSAL, Salamanca), Borja Aguinagalde (Servicio de Cirugía Torácica, Hospital Universitario de Donostia, San Sebastián-Donostia), Sergio Amor-Alonso (Servicio de Cirugía Torácica, Hospital Universitario Quirón salud Madrid, Madrid), Miguel Jesús Arrarás (Servicio de Cirugía Torácica, Fundación Instituto Valenciano de Oncología, Valencia), Ana Isabel Blanco Orozco (Servicio de Cirugía Torácica, Hospital Universitario Virgen del Rocío, Sevilla), Marc Boada (Servicio de Cirugía Torácica, Hospital Clinic de Barcelona, Instituto Respiratorio, Universidad de Barcelona, Barcelona), Isabel Cal (Servicio de Cirugía Torácica, Hospital Universitario La Princesa, Madrid), Ángel Cilleruelo Ramos (Servicio de Cirugía Torácica, Hospital Clínico Universitario, Valladolid), Elena Fernández-Martín (Servicio de Cirugía Torácica, Hospital Clínico San Carlos, Madrid), Santiago García-Barajas (Servicio de Cirugía Torácica, Hospital Universitario de Badajoz, Badajoz), María Dolores García-Jiménez (Servicio de Cirugía Torácica, Hospital Universitario de Albacete, Albacete), José María García-Prim (Servicio de Cirugía Torácica, Hospital Universitario Santiago de Compostela, Santiago de Compostela), José Alberto García-Salcedos (Servicio de Cirugía Torácica, Hospital Universitario 12 de Octubre, Madrid), Juan José Gelbenzu-Zazpe (Servicio de Cirugía Torácica, Complejo Hospitalario de Navarra, Pamplona), Carlos Fernando Giraldo-Ospina (Servicio de Cirugía Torácica, Hospital Regional Universitario, Málaga), María Teresa Gómez Hernández (Servicio de Cirugía Torácica, Hospital Universitario de Salamanca, Universidad de Salamanca, IBSAL, Salamanca), Jorge Hernández (Servicio de Cirugía Torácica, Hospital Universitario Sagrat Cor, Barcelona), Jennifer D. Illana Wolf (Servicio de Cirugía Torácica, Hospital Puerta del Mar, Cádiz), Alberto Jáuregui Abularach (Servicio de Cirugía Torácica, Hospital Universitario Vall d’Hebron, Barcelona), Unai Jiménez (Servicio de Cirugía Torácica, Hospital Universitario Cruces, Bilbao), Iker López Sanz (Servicio de Cirugía Torácica, Hospital Universitario de Donostia, San Sebastián- Donostia), Néstor J. Martínez-Hernández (Servicio de Cirugía Torácica, Hospital Universitario La Ribera, Alcira, Valencia), Elisabeth Martínez-Téllez (Servicio de Cirugía Torácica, Hospital Santa Creu y Sant Pau, Universidad Autónoma de Barcelona, Barcelona), Lucía Milla Collado (Servicio de Cirugía Torácica, Hospital Arnau de Vilanova, Lleida), Roberto Mongil Poce (Servicio de Cirugía Torácica, Hospital Regional Universitario, Málaga), Francisco Javier Moradiellos-Díez (Servicio de Cirugía Torácica, Hospital Universitario Quirón salud Madrid, Madrid), Ramón Moreno-Basalobre (Servicio de Cirugía Torácica, Hospital Universitario La Princesa, Madrid), Sergio B. Moreno Merino (Servicio de Cirugía Torácica, Hospital Universitario Virgen Macarena, Sevilla), Florencio Quero-Valenzuela (Servicio de v, Hospital Virgen de las Nieves, Granada), María Elena Ramírez-Gil (Servicio de Cirugía Torácica, Complejo Hospitalario de Navarra, Pamplona), Ricard Ramos-Izquierdo (Servicio de Cirugía Torácica, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona), Eduardo Rivo (Servicio de Cirugía Torácica, Hospital Universitario Santiago de Compostela, Santiago de Compostela), Alberto Rodríguez-Fuster (Servicio de Cirugía Torácica, Hospital del Mar. I M I M (Instituto de investigación médica Hospital del Mar, Barcelona), Rafael Rojo-Marcos (Servicio de Cirugía Torácica, Hospital Universitario Cruces, Bilbao), David Sánchez-Lorente (Servicio de Cirugía Torácica, Hospital Clinic de Barcelona, Instituto Respiratorio, Universidad de Barcelona, Barcelona), Laura Sánchez Moreno (Servicio de Cirugía Torácica, Hospital Universitario Marqués de Valdecilla, Santander), Carlos Simón (Servicio de Cirugía Torácica, Hospital Universitario Gregorio Marañón, Madrid), Juan Carlos Trujillo-Reyes (Servicio de Cirugía Torácica, Hospital Santa Creu y Sant Pau, Universidad Autónoma de Barcelona, Barcelona), Cipriano López García (Servicio de Cirugía Torácica, Hospital Universitario de Badajoz, Badajoz), Juan José Fibla Alfara (Servicio de Cirugía Torácica, Hospital Universitario Sagrat Cor, Barcelona), Julio Sesma Romero (Servicio de Cirugía Torácica, Hospital General Universitario de Alicante, Alicante) and Florentino Hernando Trancho (Servicio de Cirugía Torácica, Hospital Clínico San Carlos, Madrid).