Introduction
Complicated pneumonia is a serious disease with high morbidity and mortality, and Stevens-Johnson syndrome, though sometimes also related to other causes, may be its most dramatic complication, clinically speaking. Stevens-Johnson syndrome is rare, with between 1.2 and 6 cases yearly per 1 000 000 inhabitants,1 and certain features of the disease are poorly defined, a situation that conditions the study of a clinical picture characterized by generalized exanthema accompanied by high fever, catarrhal symptoms and the involvement of two or more mucosal tissues with their corresponding signs and symptoms (conjunctival injection, photophobia, odynophagia, itching, dysuria, etc.). If the condition progresses rapidly and leads to significant skin necrosis, Stevens-Johnson syndrome may become a serious, even fatal, complication of atypical pneumonia.
Clinical observations
We describe the case of a 30-year-old woman who went to the emergency room complaining of fever and general health deterioration. Relevant medical history included only that she had had tonsils and polyps removed in childhood due to frequent upper respiratory tract infections and that one infection was followed by self-limited exanthema. For five days before admission, the patient´s family had been experiencing a cold epidemic with rhinorrhea, dry cough and mild wheezing and she said her health deteriorated progressively, with headache, earache, pain on swallowing, nausea without vomiting, constipation, pleuritic-type bilateral chest pain, and slight dyspnea. Despite initiation of antibiotic therapy with amoxicillin-clavulanic acid, her symptoms worsened with liquid stools 8 times in 24 hours and fever, leading her to go to the emergency room.
Physical examination showed her to be in a generally deteriorated state, with a temperature of 38.1 ºC, tachycardia of 118 beats/min, a respiratory rate of 24 breaths/min and oxygen saturation of 92% on room air. Small pulpy hemorrhagic plaques were found on the soft palate, with no swollen glands in palpable areas. Auscultation revealed crackles in both pulmonary bases and more serious crepitations on the left. Blood tests showed the following: total leukocyte count 7x109 cells/L, neutrophils 82.5%, and slight anemia (red cells 11.1 g/dL, hematocrit 33.6%, and normal cell volume). Further biochemical testing showed normal ion levels but elevated transaminases (aspartate aminotransferase 57 IU/L, alanine transaminase [ALT] 56 IU/L) and elevated lactate dehydrogenase (LDH; 285 IU/L). Hemostasis testing showed a fibrinogen level of 851 mg/dL and arterial blood gas samples showed PO2 at 55 mm Hg, PCO2 at 33 mm Hg, pH at 7.53, bicarbonate concentration at 27 mmol/L, and SaO2 at 92% on room air. A chest x-ray revealed increased opacities in the middle right and lower left areas indicating bilateral pneumonic infiltrates.
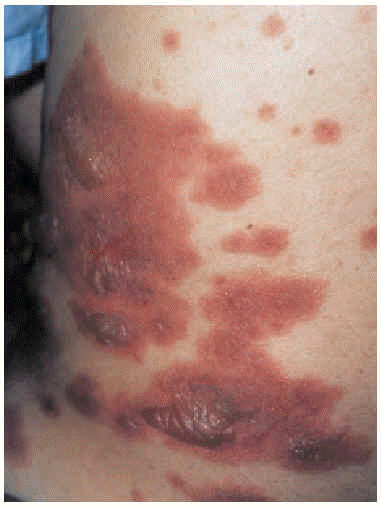
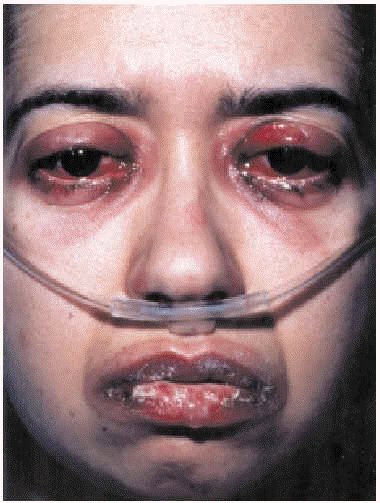
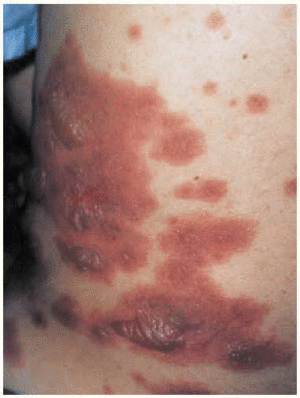
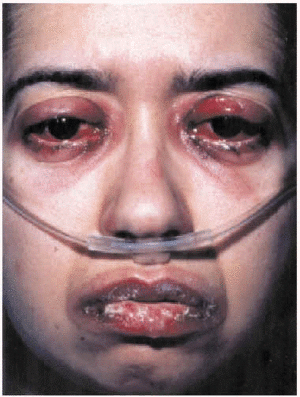
The patient was admitted to the respiratory medicine ward with a diagnosis of bilateral atypical pneumonia with partial respiratory insufficiency and respiratory alkalosis. Intravenous antibiotic therapy was begun with a third generation cephalosporin and a macrolide. Treatment also included antipyretics, analgesics, bronchial aspiration, fluid replacement, and oxygen support with inspired oxygen fraction set at 0.35. On the second day on the ward, although respiratory improvement was evident and no progression was evident on a chest x-ray, generalized rapidly progressing cutaneous exanthema developed (Figure 1) in the form of red, erythematous plaques with papulovesicular eruptions at the center (target lesions). Bilateral mucopurulent conjunctivitis and generalized mucositis in the oropharyngeal, digestive, urethral and vaginal regions were also found. After consultation with the attending ophthalmologist and dermatologist, and based on a clinical diagnosis of Stevens-Johnson syndrome probably due to Mycoplasma pneumoniae infection, the patient was started on mydriatic eyewashes of tobramycin and glucocorticoids, antiseptic mouthwashes, local treatments of sulfated water and diluted chlorhexidine, and systemic tetracycline. However, cutaneous lesions spread and became severe (Figure 2), involving all digestive mucosa to a degree that affected liquid and food intake (signs of dysphagia and diarrhea), urinary mucosa (dysuria, polyuria, vesicular tenesmus with urgency) as well as vaginal and conjunctival mucosa, although the patient´s general condition was scarcely affected by temperature peaks.
Fig. 1. Generalized cutaneous exanthema consisting of pruritic red erythematous plaques with papulovesicular eruptions at their center (bullous target lesions) on the patient's back.
Fig. 2. Mucopurulent conjunctivitis and oral mucositis.
Five days after admission, systemic quinolones were withdrawn given the possibility that treatment with beta-lactams might be causing the clinical picture, and intravenous glucocorticoid treatment was started. The patient was transferred to the serious burns unit because lesions had spread over 15% of her body. Blood tests showed potassium levels at 2.7 mEq/L, ALT at 75 IU/L, LDH at 291 IU/L and a hematocrit of 27.9% with a red cell count of 9.1 g/dL; other parameters were within normal ranges. After 10 days in the burns unit under treatment with silver sulfadiazine cream, the exanthema had improved considerably and the patient was transferred back to the respiratory medicine ward. When she was discharged 18 days after admission she was asymptomatic and the chest x-ray was normal. Twenty-eight days after discharge she was in good health and clinical recovery was complete except for hyperpigmented cutaneous lesions.
During her hospital stay, cultures were ordered on the following samples: urine, stool, throat swab (bacteriology and virology), mouth ulcer swab (virology), conjunctival swab, skin exudate and sputum. We also ordered tests for parasites in stool (Graham test), for Legionella pneumoniae antigen in urine, for Chlamydia pneumoniae, Chlamydia psittaci, Coxiella burnetii (antiphase I and II antibodies) in serum, as well as Ziehl-Neelsen stains of sputum (x2); all results were negative. The following results were also available: serology for measles virus (immunoglobulin [Ig] G enzyme-linked immunosorbent assay [ELISA]: 1/5000; IgM ELISA: negative) and for Epstein-Barr virus (IgG virus capsid antigen [VCA] ELISA: positive; IgM VCA ELISA: negative). The IgM ELISA for M. pneumoniae was negative at the time of admission, became positive after 2 months and negative at 6 months, whereas the IgG ELISA, which was also negative at admission and at 2 months, became positive 6 months after admission, providing confirmation of the etiologic diagnosis. The skin biopsy gave results that were characteristic of erythema multiforme, supporting the initial clinical diagnosis and ruling out staphylococcal scalded skin syndrome.
Discussion
Approximately a quarter of community-acquired pneumonias are caused by microorganisms responsible for atypical pneumonia,2-4 mainly M. pneumoniae,3 a pathogen that can affect any age group but that most often strikes adolescents and young adults.
After an incubation period of 2 to 3 weeks, a clinical picture of atypical pneumonia may develop associated or not with other infections of the upper respiratory tract and ear, such as tracheitis, bronchitis, myringitis and otitis, that may also be attributable directly to M. pneumoniae. The etiologic diagnosis is usually made through serum testing using complement-fixation techniques or ELISA.2,5 A great variety of extrapulmonary complications have been described: meningoencephalitis; aseptic meningitis; encephalitis; transverse myelitis; hemolytic anemia; thrombocytopenia; disseminated intravascular coagulation; myopericarditis; congestive heart failure; coronary blockage; arthritis and cutaneous lesions, especially exanthemas (macular, maculopapular, morbilliform, papulovesicular or petechial); Stevens-Johnson syndrome; erythema nodosum; and wheals.
M. pneumoniae is the most frequent cause of erythema in pneumonia8 and it is also the respiratory pathogen that most often causes Stevens-Johnson syndrome. Some authors suggest that 7% of cases of M. pneumoniae infection develop into this syndrome,2 which can also appear without associated pneumonia.4,6 Although Stevens-Johnson syndrome has been linked to increased sensitivity to antibiotics in the presence of M. pneumoniae infection,2 in reality we have a poor understanding of the pathogenesis of an entity that affects young people more frequently than the elderly and men more than women and that has recently been differentiated from erythema multiforme major on the basis of its epidemiology, etiology and severity.9 Stevens-Johnson syndrome is characterized by a generalized exanthema with bullous target lesions (macules with active centers and borders that migrate centrifugally to form bullae with erythematous halos), and atypical squamous lesions usually affecting the face and upper trunk. High fevers and cold symptoms are present and two or more mucosa are involved, with corresponding symptoms: conjunctival injection, photophobia, odynophagia, pruritus, dysuria, etc. Diagnosis is based on clinical presentation and confirmed by skin biopsy, although the cause of infection must be confirmed serologically.5 The most frequently reported causes of Stevens-Johnson syndrome are medication, atypical pneumonia due to M. pneumoniae, and herpes virus, in that order.8 In the case we report, bacterial infection rather than medication or viral infection was identified as the cause based on serological confirmation and other evidence, such as the appearance of exanthema during the first week after the onset of symptoms and the rapid progression of lesions.1
The seriousness of Stevens-Johnson syndrome is evident in the speed with which it takes hold and the extension of skin necrosis, together with frequently developing complications such as dehydration, prerenal acute renal failure, interstitial pneumonitis, adult respiratory distress syndrome, and particularly, septic shock from overlying infection. The last complication is the most frequent cause of death in patients with Stevens-Johnson syndrome.
Acknowledgments
We would like to thank the staff of the serious burns unit and the departments of ophthalmology, dermatology, and microbiology at our hospital for their interest in teaching us and for their clinical support.
Correspondence: Dr. M. Modesto Alapont.
Servicio de Neumología. Hospital Universitario La Fe. Avda. Campanar, 21.
46009 Valencia. Spain.
E-mail: manumalap@ono.com
Received for publication November 20, 2002.
Accepted for publication December 12, 2002.