Pneumomediastinum is defined by the presence of free air in the mediastinum, and is usually accompanied by subcutaneous emphysema. It is classified as spontaneous when there is no clear cause, and traumatic when it occurs as the result of a mediastinal intervention, perforation or trauma. Spontaneous pneumomediastinum occurs when the terminal alveoli are ruptured by tracheal hyperinflation.1 One of the lesser known causes of spontaneous pneumomediastinum is Hamman’s syndrome (described by Louis Hamman in 1939), which refers to spontaneous pneumomediastinum associated with subcutaneous emphysema occurring in the postpartum period.2,3 It is a rare entity, with only 200 cases being described in the literature up to 1994.4 We report the case of a 29-year-old woman who presented pneumomediastinum and subcutaneous emphysema after a vaginal delivery.
Our patient was a 29-year-old woman, primigravida, 40 + 3 weeks of gestation, with no significant medical history, who presented in the emergency department with a 12-h history of irregular uterine contractions every 15 min. She had no vaginal bleeding and the amniotic sac was intact. Her pregnancy had been appropriately monitored and incident-free. At the onset of labor, an abdominal ultrasound and fetal cardiotography showed no abnormalities. The patient had a spontaneous vaginal delivery with cephalic presentation, and gave birth to a boy weighing 3.2 kg with an Apgar score of 10 in the first minute. Labor lasted 10 h and there were no complications.
Eighteen hours after delivery, the patient complained of odynophagia and a sensation of crackling in the cervical region, without dyspnea. Her blood pressure was 126/73 mmHg, heart rate 80 bpm, and oxygen saturation 98% breathing room air. Pulmonary auscultation was normal. A chest X-ray showed bilateral subcutaneous emphysema in both clavicular and cervical regions, with no evidence of pneumothorax. A blood test showed hemoglobin 11.8 g/dL, 113,000 platelets/mm3, 12,700 leukocytes/mm3 (82.3% neutrophils). Renal function, ions, and liver function tests were normal. Chest computed tomography (CT) showed deep and extensive cervical subcutaneous emphysema and extensive pneumomediastinum. Mediastinitis, free fluid, and esophageal rupture were ruled out. A diagnosis of spontaneous pneumomediastinum secondary to labor was made, and we decided on conservative management. Subsequent progress was favorable, and the patient was asymptomatic with complete resolution of the lesions within 2 weeks of follow-up.
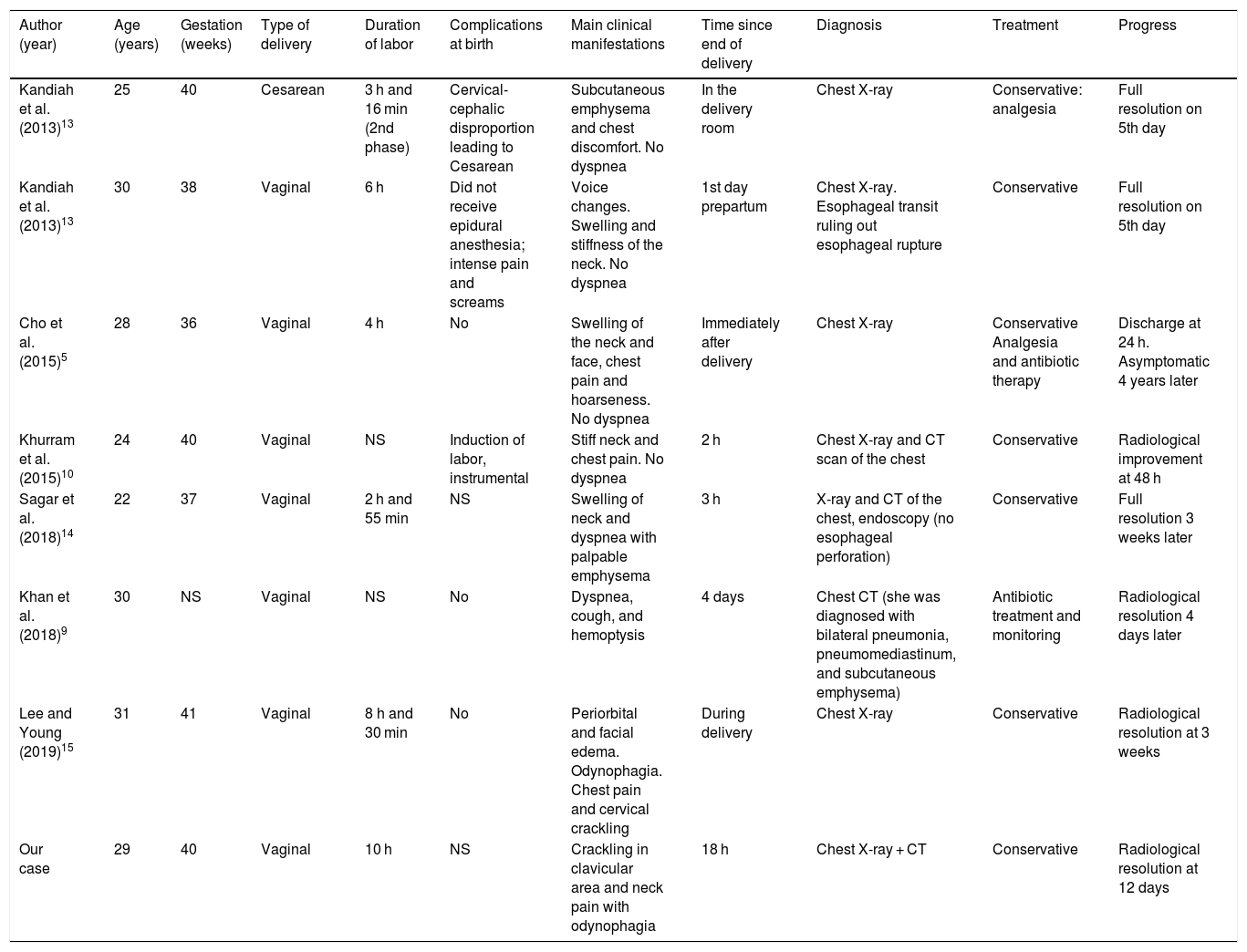
Hamman’s syndrome, described in 1939, is defined by the association of extensive subcutaneous emphysema and pneumomediastinum in the immediate postpartum period. It is a rare complication associated with labor, and occurs at an incidence of approximately 1 in 100,000 births,1,5 although it has also been described in association with other processes not limited to the postpartum period, such as screaming, cough, hyperemesis, respiratory infections, or physical exertion.6,7 It is more frequent in young primiparous women and is associated with the delivery of large babies.8 It has been posited that it can also occur during the perinatal period in patients with hyperemesis gravidarum. However, most cases occur in the second stage of labor in primigravidas who have a prolonged and difficult labor, due to efforts made during the Valsalva maneuvers. Symptoms usually appear in the third or fourth stages of labor.5Table 1 shows the cases of Hamman’s syndrome published in the past 10 years.
Cases of Hamman’s syndrome published in the literature over the past 10 years.
| Author (year) | Age (years) | Gestation (weeks) | Type of delivery | Duration of labor | Complications at birth | Main clinical manifestations | Time since end of delivery | Diagnosis | Treatment | Progress |
|---|---|---|---|---|---|---|---|---|---|---|
| Kandiah et al. (2013)13 | 25 | 40 | Cesarean | 3 h and 16 min (2nd phase) | Cervical-cephalic disproportion leading to Cesarean | Subcutaneous emphysema and chest discomfort. No dyspnea | In the delivery room | Chest X-ray | Conservative: analgesia | Full resolution on 5th day |
| Kandiah et al. (2013)13 | 30 | 38 | Vaginal | 6 h | Did not receive epidural anesthesia; intense pain and screams | Voice changes. Swelling and stiffness of the neck. No dyspnea | 1st day prepartum | Chest X-ray. Esophageal transit ruling out esophageal rupture | Conservative | Full resolution on 5th day |
| Cho et al. (2015)5 | 28 | 36 | Vaginal | 4 h | No | Swelling of the neck and face, chest pain and hoarseness. No dyspnea | Immediately after delivery | Chest X-ray | Conservative Analgesia and antibiotic therapy | Discharge at 24 h. Asymptomatic 4 years later |
| Khurram et al. (2015)10 | 24 | 40 | Vaginal | NS | Induction of labor, instrumental | Stiff neck and chest pain. No dyspnea | 2 h | Chest X-ray and CT scan of the chest | Conservative | Radiological improvement at 48 h |
| Sagar et al. (2018)14 | 22 | 37 | Vaginal | 2 h and 55 min | NS | Swelling of neck and dyspnea with palpable emphysema | 3 h | X-ray and CT of the chest, endoscopy (no esophageal perforation) | Conservative | Full resolution 3 weeks later |
| Khan et al. (2018)9 | 30 | NS | Vaginal | NS | No | Dyspnea, cough, and hemoptysis | 4 days | Chest CT (she was diagnosed with bilateral pneumonia, pneumomediastinum, and subcutaneous emphysema) | Antibiotic treatment and monitoring | Radiological resolution 4 days later |
| Lee and Young (2019)15 | 31 | 41 | Vaginal | 8 h and 30 min | No | Periorbital and facial edema. Odynophagia. Chest pain and cervical crackling | During delivery | Chest X-ray | Conservative | Radiological resolution at 3 weeks |
| Our case | 29 | 40 | Vaginal | 10 h | NS | Crackling in clavicular area and neck pain with odynophagia | 18 h | Chest X-ray + CT | Conservative | Radiological resolution at 12 days |
CT: computed tomography; h: hours; min: minutes; NS: not specified.
The pathophysiology of Hamman’s syndrome is based on the rupture of alveoli due to a marginal increase in intraalveolar pressure with sustained Valsalva maneuvers (forced expiration against a closed glottis) associated with coughing, vomiting, screaming, and pushing during delivery. Intrathoracic pressure can rise to levels of 50 cmH2O.9 This increase in pressure in the presence of reduced vascular caliber establishes a pressure gradient in the vascular sheath along which air can dissect the mediastinum, with subsequent migration of air to the subcutaneous planes.
The most common clinical presentation of Hamman’s syndrome consists of retrosternal chest pain, shortness of breath, facial or neck pain, odynophagia, and dysphagia. Since chest pain during childbirth can have different etiologies, urgent conditions such as pulmonary embolism, amniotic fluid embolism, myocardial infarction, pneumothorax, and aortic dissection, must be first be ruled out.3 In the case of hyperemesis, esophageal rupture should be ruled out, since it can be precipitated by the same factors.
Chest X-ray is the initial diagnostic technique. CT is considered the gold standard to rule out mediastinal air because it can detect small amounts of air that are not visible on chest X-ray, which occurs in up to 30% of cases.1,10
Recurrence of Hamman’s syndrome is rare and patients usually respond favorably to conservative treatment, consisting essentially of on-demand analgesia, oxygen therapy when necessary, and rest. Patients can be safely discharged to home with analgesic treatment. In very exceptional cases, when pneumothorax is also present, placement of a chest tube may be necessary.11,12
In conclusion, spontaneous pneumomediastinum, despite being a rare disease, should be considered in the differential diagnosis of chest pain in the immediate postpartum period. Chest X-ray is a useful tool but it is not always diagnostic, while chest CT is the diagnostic test of reference. Progress is favorable, recurrence is uncommon, and management is often conservative.
Please cite this article as: García-García A, et al. Neumomediastino y enfisema subcutáneo espontáneo: síndrome de Hamman. Arch Bronconeumol. 2019;55:661–663.