Spirometry is the main pulmonary function test and is essential for the evaluation and monitoring of respiratory diseases. Its utility transcends the field of Respiratory Medicine, is becoming increasingly important in primary care and applications have even been described outside the field of respiratory diseases. This document is therefore intended to serve as support for all health professionals who use spirometry, providing recommendations based on the best scientific evidence available.
An update of the indications and contraindications of the test is proposed. The document sets out recommendations on the requirements necessary for conventional spirometers and portable office equipment, as well as on spirometer hygiene and quality control measures. Spirometric parameters that must be considered, performance of manoeuvres, criteria for acceptability and repeatability of measurements and their quality control are defined. A proposal is also established for presentation of the results and an evaluation and interpretation is proposed according to information generated in recent years. Finally, lines of adaptation and integration of spirometry in the field of new technologies are considered.
La espirometría es la principal prueba de función pulmonar, y resulta imprescindible para la evaluación y el seguimiento de las enfermedades respiratorias. Su utilidad trasciende el ámbito de la neumología, adquiere una creciente importancia en atención primaria e incluso se han descrito aplicaciones fuera del campo de las enfermedades respiratorias. Por ello, este documento pretende servir de apoyo a todos los profesionales de la salud que utilicen la espirometría, proporcionando recomendaciones basadas en las mejores evidencias científicas disponibles.
Se propone una actualización de las indicaciones y contraindicaciones de la prueba. El documento establece recomendaciones sobre los requerimientos necesarios para los espirómetros convencionales y los equipos portátiles de oficina, así como sobre las medidas de higiene y de control de calidad de los espirómetros. Se definen los parámetros espirométricos que deben ser considerados, la realización de las maniobras, los criterios de aceptabilidad y repetibilidad de las medidas y su control de calidad. También se establece una propuesta para la presentación de los resultados y se recomienda una evaluación e interpretación acorde a la información generada en los últimos años. Por último, se consideran las líneas de adaptación e integración de la espirometría en el campo de las nuevas tecnologías.
Spirometry is a basic test for the study of lung function, and its performance is necessary for the evaluation and follow-up of respiratory diseases. Its usefulness transcends the field of Respiratory Medicine, and the last few years have seen it gradually incorporated in primary care and other medical disciplines.
Aware of its importance, the first Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) guidelines were dedicated to spirometry.1 The need to incorporate technological advances and changes in the performance, evaluation and interpretation of the procedure in the time since then has created the requirement for this new version. This document includes analyses and position proposals on the characteristics and requirements of conventional spirometers and currently available portable office equipment, as well as on the required quality criteria and parameters that must be analysed. An assessment and interpretation in line with the information generated in recent years is also proposed, including lines of adaptation and integration of spirometry in the field of new technologies.
The present guidelines are aimed at all healthcare professionals who use this test, which is intended to serve as a reference for decision-making based on the best scientific evidence available.
Applications. Indications and ContraindicationsApart from its usefulness for the diagnosis and monitoring of many respiratory diseases, spirometry has other potential applications. There is evidence that determination of the lung function age can improve the success of smoking cessation,2 and that spirometry is useful for estimating the risk of lung cancer, cognitive deterioration, all-cause mortality and mortality of cardiovascular origin.3–5
The main indications for spirometry are summarised in Table 1.1,6–9 It is essential for the diagnosis and monitoring of most respiratory diseases. Furthermore, it enables the impact of diseases of other organs or systems (cardiac, renal, liver, neuromuscular, etc.) on lung function to be assessed. Thus, it should form part of any routine health examination, especially in subjects at risk of developing lung diseases. It is recommended that spirometry be systematically performed in persons over the age of 35 with a history of smoking (>10 pack-years) and any respiratory symptom (quality of evidence: moderate; strength of recommendation: strongly in favour).10
Indications for Spirometry.
| Diagnostic |
| Evaluation of respiratory symptoms or signs |
| Measurement of the effect of the disease on lung function |
| Screening of subjects at risk of lung disease, mainly: |
| - Smokers aged over 35 years and at least 10 packs-year |
| - Persistence of respiratory symptoms, including dyspnoea, cough, expectoration, wheezing or chest pain |
| - Work or occupational exposure to toxic substances that cause respiratory impairment |
| Risk evaluation for surgical procedures, especially chest or upper abdominal procedures |
| Estimation of severity and prognosis in respiratory diseases or diseases of other organs that affect respiratory function |
| Assessment of health status before beginning strenuous physical activity programmes |
| Routine physical examination |
| Monitoring |
| Evaluation of the effect of therapeutic interventions |
| Monitoring the course of diseases that affect lung function |
| Monitoring persons exposed to substances that are potentially toxic for the lungs, including drugs |
| Evaluation of deterioration/disability |
| Rehabilitation programmes |
| Evaluation of dysfunction for medical insurance and legal assessments (social security, expert reports, etc.) |
| Public health |
| Epidemiological studies |
| Generation of reference equations |
| Clinical research |
Spirometry is generally well tolerated, so there are few limitations for its performance in routine daily practice.1,6–9,11,12 Certain contraindications for spirometry have been established (Table 2) from a more detailed analysis of the frequency of developing complications in certain risk situations and their severity,13,14 differentiating the absolute contraindications, in which the test is not recommended, and the relative contraindications, which require individualised assessment of the ratio between the potential risks and expected benefits.
Contraindications of Spirometry.
| Absolute |
| Haemodynamic instability |
| Pulmonary embolism (until adequately anticoagulated) |
| Recent pneumothorax (2 weeks after re-expansion) |
| Acute haemoptysis |
| Active respiratory infections (tuberculosis, norovirus, influenza) |
| Recent myocardial infarction (7 days) |
| Unstable angina |
| Aneurism of the thoracic artery that has grown or is large in size (>6cm) |
| Intracranial hypertension |
| Acute retinal detachment |
| Relative |
| Children under 5–6 years old |
| Confused or dementia patients |
| Recent abdominal or thoracic surgery |
| Recent brain, eye or ear, nose or throat surgery |
| Acute diarrhoea or vomiting, nausea |
| Hypertensive crisis |
| Dental or facial problems that impede or make it difficult to insert and hold the mouthpiece |
In patients with pulmonary embolism, it is recommended that the test is not performed until patients have been adequately anticoagulated (usually after receiving 2 doses of low molecular weight heparin), and in patients with pneumothorax for up to 2 weeks after reaching re-expansion.13 Current evidence suggests that spirometry is safe 7 days after an uncomplicated myocardial infarction, provided that the patient remains stable.13 While unstable angina is an absolute contraindication, spirometry is occasionally required in the preoperative assessment of patients with chronic stable angina. In this case, the previous administration of sublingual nitroglycerin should be considered.13
In several studies, spirometry has been performed 2h after thoracotomy, with no notable complications.14 Nonetheless, the limitation established by the pain may determine the usefulness of data obtained. It is recommended that the test be postponed for at least one week after abdominal surgery and for 3–6 weeks after brain surgery.13 In the case of eye surgery, the examination should be delayed for 2 weeks following oculoplastic surgery, 2 months after vitreoretinal surgery (vitrectomy or glaucoma surgery) and 3 months for anterior eye segment surgery (cataracts or keratomy).15 In the case of hypertensive crisis, it is also recommended to postpone the examination until a mean arterial systolic pressure of less than 130mmHg has been reached.13
In any case, complications in forced spirometry are rare. The most common are paroxysmal coughing, bronchospasm, chest pain, dizziness, urinary incontinence or increased intracranial pressure. More rarely, the patient may suffer syncopal symptoms. The competency of the healthcare staff performing the spirometry (hereinafter technician) is essential for detecting problems and halting the test. The insistence on obtaining assessable data once the patient presents any of these complications is contraindicated, and the spirometry must be postponed for another day.
VariablesThe primary variables in forced spirometry are the forced vital capacity (FVC) and forced expiratory volume in the first second (FEV1). The FVC represents the maximum volume of air exhaled in a maximal forced expiratory manoeuvre, initiated after a maximal inspiratory manoeuvre, expressed in litres. The FEV1 corresponds to the maximal volume of air exhaled in the first second of the FVC manoeuvre, also expressed in litres. In turn, the FEV1/FVC ratio shows the relationship between both parameters. It should not be confused with the Tiffeneau index, which is defined as the ratio between the FEV1 and the slow vital capacity (VC).
The FEV6, or maximal volume of air exhaled in the first 6s of the FVC manoeuvre, and the FEV1/FEV6 ratio may constitute alternative parameters to the previous in the interpretation of the spirometry, especially when simplified portable equipment is used.16 The FEVt corresponds to the maximal volume of air exhaled in time “t”. It has been suggested that in children who are unable to perform a forced manoeuvre for one second, the FEV0.5 or FEV0.75 may be used as equivalents of the FEV1,17 although there are insufficient data in this respect.
In addition to volumes, various flows must also be considered. The mid forced expiratory flow (FEF25%–75% or MMEF) is defined as the flow measured between 25% and 75% of the forced expiratory manoeuvre (expressed in ls−1). The peak expiratory flow (PEF) is obtained from the peak value in the expiratory loop of the flow-volume curve and is also expressed in litres s−1. Instantaneous expiratory flows (FEFx%) refer to the flow when the corresponding percentage of the FVC (x %) has been exhaled. The most widely used are the FEF25%, FEF50% and FEF75% (ls−1). Among the inspiratory parameters, the following should be considered: the inspiratory forced vital capacity (IFVC), or maximal volume of air inspired, in a maximal forced inspiratory manoeuvre initiated after a maximal expiratory manoeuvre; the forced inspiratory volume in the first second (FIV1), or maximal volume of air inspired in the first second of the IFVC manoeuvre; the mid inspiratory flow (FIF25%–75% or MMIF), or flow between 25% and 75% of the forced inspiratory manoeuvre; the peak inspiratory flow (PIF), or peak flow in the forced inspiratory manoeuvre; and the instantaneous inspiratory flows (FIF25%, FIF50% and FIF75%), or inspiratory flows when 25%, 50% or 75% of the IFVC has been inhaled, respectively.
In unforced or slow spirometry (there are no standardised criteria to define an unforced manoeuvre, but it should be slower than a forced manoeuvre), the following should be measured: the vital capacity (VC), or maximal volume of air exhaled in an unforced expiratory manoeuvre initiated after a maximal inspiratory manoeuvre, and the inspiratory capacity (IC), which is the maximal inspiration taken at the end of the tidal expiration and corresponds to the sum of the circulating or tidal volume (VT) and inspiratory reserve volume (IRV).18 In some circumstances it is also useful to determine the expiratory reserve volume (ERV).
EquipmentPhysical SpaceSufficient space is required to be able to position the patient comfortably, taking into account that it may be necessary to handle a wheelchair. Therefore, the minimum recommended space is 2.5×3m, with 120cm wide doors. In laboratories where spirometry can be performed in decubitus, the size of the area should allow positioning of a hospital bed.19 The spirometer should be placed on a table or countertop which allows the technician to work in various positions with respect to the patient. The space should have sufficient storage for all the material.
A certified stadiometer and scales are essential. A thermometer, barometer and hygrometer, which must pass regular accuracy or calibration checks, are required in equipment that does not incorporate these devices.
EquipmentThere are 2 broad classes of spirometers: closed circuit and open circuit. Within the closed spirometers are wet and dry spirometers, consisting of an air collection system which may be either a piston (chamber containing a moveable plunger) or bellows (more manageable) and a recording system mounted on a support that moves at the desired rate. Most modern spirometers can also derive the flow value from the volume measured.
The most widely used spirometers at present are those known as open systems, as they do not have a hood or similar recipient to collect the air.9,20 These measure the air flow directly and, integrating the signal, calculate the volume. There are different systems, but the best known are pneumotachographs, which measure the difference in pressure generated on passing a laminar flow through a known resistance. Most equipment currently uses a resistance, either metallic and heated to prevent condensation, or made of synthetic tissues. Since the head transforms the turbulent flow that passes through into a laminar flow, the pressure difference between the ends of the pneumotachograph is directly proportional to the flow. A pressure transducer transforms the differential pressure signal into an electrical signal, which is then amplified and processed. The electronic integration of the flow value provides the volume moved.6
There are other open systems that use other principles.2,20 The most widely used at present is the turbine flow metre, which is based on the fact that the rate at which the blades turn, recorded by optical sensors, is proportional to the flow that passes through the device.20 Hot wire spirometers, or thermistors, have a metal wire in their head (generally platinum) heated to a constant temperature by an electric current. As the air flow passes, the wire cools and the flow is calculated. Ultrasound spirometers are based on one of these properties, so that when they form a certain angle with the direction of the flow, the ultrasounds that go in the same direction as the flow take less time to reach the receptor than those that go in the opposite direction. The greater the time difference the higher is the flow.20 The choice of equipment depends on the type of use, but can also vary depending on other circumstances, such as technological development or the cost. Closed systems can generally be considered more reliable, as they are precise and accurate over a range of volumes, but they also have the disadvantage of being larger, have more inertia and are more expensive. One of their major problems is difficulty in cleaning and sterilisation.21
The open systems, however, are easy to clean and have a very low risk of contamination.21 They are precise and accurate, once the necessary adjustments have been made, but require verification of satisfactory calibration and measurement conditions. Although turbine spirometers were initially blamed for non-linearity problems in the response and a certain degree of inertia, extremely reliable turbine spirometers are available today.20 To these characteristics we can add their stability, little or no need for calibration (although this should be checked regularly), ease of cleaning and low price. Furthermore, disposable turbines have recently been marketed.
Minimum SpecificationsSome minimal recommendations are established for all equipments (Table 3). The main specifications that all spirometers must meet are that the total resistance (including the entire tubing, mouthpiece and filters) for an air flow of 14ls−1 is less than 1.5cmH2Ol−1s−1 (0.15kPal−1s−1), that it measures a volume greater than 8l with an accuracy of ±3% or ±50ml and that it reaches a flow measurement range of ±14ls−1, with a sensitivity of 200ml/s.7,9,20 It is also recommended that it can record an expiration time for the forced manoeuvre of at least 15s.
Minimal Specifications that a Spirometer Must Meet and for the Spirometry Graph.
| Parameter | Range | Precision, BTPS | Range flow, ls−1 | Time, s | Resistance | Calibration signal |
| VC | 0.5–8l | ±3% of the measurement or ±0.05 l, whichever is greater | 0–14 | 30 | 3l syringe | |
| FVC | 0.5–8l | ±3% of the measurement or ±0.05 l, whichever is greater | 0–14 | 15 | <1.5cmH2Ol−1s−1 (0.15kPal−1s−1) | 3l syringe, 24 curves |
| FEV1 | 0.5–8l | 0–14 | 1 | <1.5cmH2Ol−1s−1 (0.15kPal−1s−1) | 3l syringe, 24 curves | |
| PEF | ±10% of the measurement or ±0.30 l, whichever is greater | 0–14 | ||||
| Instantaneous flows | ±5% of the measurement or ±0.20l, whichever is greater | 0–14 | <2.5cmH2Ol−1s−1. a 600lm−1 (0.25kPal−1s−1) |
| Graphical Representation | ||||
| Parameter | Screen | Paper | ||
| Resolution | Scale | Resolution | Scale | |
| Volume | 0.050l | 5mml−1 | 0.025l | 10mml−1 |
| Flow | 0.200ls−1 | 2.5mml−1s−1 | 0.100ls−1 | 5mml−1s−1 |
| Time | 0.2s | 10mms−1 | 0.2s | 20mms−1 |
BTPS, body temperature and pressure saturated with water vapour; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; PEF, peak expiratory flow; VC, slow vital capacity.
To control the quality of the test, it must be viewed in real time in order to evaluate the quality of the manoeuvres and detect artefacts. Therefore, the display should be of sufficient size and resolution (Table 3). It is recommended that the manufacturer includes a period of at least 0.25s before starting the manoeuvre (time zero) to be able to assess the back extrapolated volume; it is also advisable to view the end of the manoeuvre clearly to ensure that the emptying time has been sufficient.
The report should have flow-volume and volume-time graphs, as these provide complementary information. A volume scale of ≥10mm/l and time scale of ≥20mm/s are recommended for the graph of the report (Table 3).
Hygiene and Infection ControlClosed equipment is more complex when it comes to cleaning and disinfection than open spirometers. The use of individual mouthpieces is recommended, either disposable or sterilised, with a disposable antimicrobial filter in situations where there is a risk of contaminating the equipment. This also helps to keep the membrane of the pneumotachograph clean and thus maintain its stability.21 Cleaning and disinfection of the membranes and flow metre parts are recommended if it is not disposable and the patient inhales from the equipment. If anti-bacterial filters are used, this disinfection can be daily or when contamination is suspected. In cases with a high risk of transmission, thorough cleaning and disinfection or complete sterilisation of the flow metre may be necessary.21 Periodically, and when there is biological material (blood stains or secretions), the surface of the equipment should be cleaned according to its characteristics and manufacturer's instructions. If antimicrobial filters are used, calibration or verification of the equipment should be performed with the filter inserted in the calibration syringe. Clean nose clips should be used for each patient. These can be disposable, or a set of nose clips that are sufficient for everyday work, and which can be cleaned with soap and water at the end of the day, can also be used.
Quality Control: Volume and Flow CalibrationsIn addition to the auto-calibration procedures that may be built into the spirometer, it must be possible to check the calibration of the apparatus by applying external signals that should resemble the actual biological signal from the forced respiration, both in terms of the magnitude of flows and in volumes and times. In this respect, certified syringes of various litres provide an adequate signal volume and the flow generators are used to assess the precision and errors in the flow measurement. The syringe should be kept in the same room where the calibration is performed and should not be exposed to heat or cold sources.
A certified 3l syringe should be used to confirm that the precision in the volume measurement remains within the recommended range (±3%). The calibration syringes should have a precision of ±15ml or ±0.5% of the complete scale, and must be checked periodically for leaks and to verify their stability. Under normal working conditions, the volume calibration using a certified 3l syringe should be performed daily in pneumotachographs and weekly in dry closed circuit spirometers. In the case of disposable sensors, calibration should be performed every time they are used if they are not pre-calibrated (factory-calibrated), in which case it should be done daily.
Calibration of the flow rate is more complex, as performing it precisely requires specialised equipment (explosive decompressor). The best alternative is to apply the manual syringe at different flow rates, from very low to very high, trying to simulate the normal patient range. Calibration at different flow rates is recommended daily, and whenever there are doubts that the equipment is measuring correctly. It is recommended that this quality control procedure be included in computer calibration systems.
In modern computer-controlled equipment, in principle, the time metre does not need to be checked, but in the case of spirometers with a timer or chronometer for obtaining the flow-time curve, their working order should be verified monthly using a manual chronometer. In general, the error in the time measurement should be less than 2%.
An additional quality control procedure is to use biological controls, i.e. healthy, non-smokers whose cooperation can be attained and who perform the spirometry correctly, easily and with little variablility.22 The use of biological controls is recommended monthly or when there are doubts, and the measurement is not expected to vary by more than 5% or 100ml.23
MaintenanceMaintenance should be carried out in accordance with the instructions provided by the equipment manufacturer. A log book should be kept where changes in the daily calibrations, as well as adjustments or repairs carried out, are recorded.
Technician TrainingSpirometry is an apparently simple technique but is complex to execute, and requires experts for its performance and quality control. Recently, the European Respiratory Society (ERS) has designed a training procedure, the European Spirometry Driving License, which was launched in 2012.24 Until its introduction, it is advisable to attend structured courses. At least 3 months of specific experience are required, supervised by expert technicians, to ensure minimum competence in carrying out the test. Knowledge of the apparatus and solutions to common problems requires up to a year of experience. According to American Thoracic Society (ATS) and ERS recommendations, the technician must have sufficient training to understand the technical and physiological bases of the tests, as well as the common signs of respiratory diseases and the management of data collected by the lung function equipment. Since the bronchodilation procedure includes the administration of drugs, the technician should have a healthcare qualification or if not, they should be directly supervised by a Healthcare graduate with formation in lung function, who will assume direct responsibility. Staff in charge of spirometry should have continuity, the possibility of periodic retraining and contact with a reference laboratory.
ProcedurePreparation of the Patient and EquipmentOn presenting in the laboratory, patients should bring (or it should be listed in the electronic diary) a referral slip with the required tests. When the appointment is made, they should be given written instructions which state the guidelines for withdrawal of bronchodilators (Table 4), as well as the importance of abstaining from smoking or doing any physical exercise in the hours prior to the test.
Recommended Waiting Time for Performing Forced Spirometry After Having Taken Bronchodilator Medication.
| Drug | Hours |
| Short-acting β2-adrenergic agonists | 6 |
| Long-acting β2-adrenergic agonists | 12 |
| Ultra long-acting β2-adrenergic agonists | 24 |
| Short-acting anticholinergics | 6 |
| Long-acting anticholinergics | 24 |
| Sustained release theophyllines | 36–48 |
Before beginning the examination, the test should be explained to the patient, stressing the importance of their collaboration. They should also be asked about the withdrawal of drugs, possible contraindications or infectious diseases that require special measures (in which case it is recommended that the spirometry be postponed until the end of the day, just before cleaning the equipment, and that antimicrobial filters are used).
The patient should be measured barefoot with their back placed firmly against the stadiometer, weighed wearing light clothing and asked their date of birth to calculate their age on the day on which the test is performed. In the case of chest deformities, the span should be measured (crossed arms from middle finger to middle finger), estimating the height from the following ratio: height=span/1.06.25 Another possibility is to use the equations proposed by Parker et al.,25 which are valid for different ethnic groups.
The test should be performed with the individual sitting upright, without crossing their legs or wearing tight-fitting clothing. In the case of children, it can be performed either sitting or standing, noting the way in which it is carried out and always using the same procedure for the same individual. During the manoeuvre, the back must be supported on the backrest, ensuring that the patient does not lean forward while performing the test. Dentures do not need to be removed, unless they make it difficult to perform the manoeuvres.
The use of a nose clip in forced spirometry is controversial, although it is essential for measuring the VC, to prevent possible leakage due to nasal respiration. Although some authors have not identified differences between manoeuvres carried out with or without a nose clip,26,27 their use is recommended in adults.9
Before beginning the examination, check that the calibration has been verified on the day of the test. If a meteorological station incorporated in the equipment is used, the atmospheric pressure, humidity and ambient temperature should be entered or verified.
Description of the ManoeuvreBefore beginning, the subject must be given precise, clear and concise instructions. After placing the mouthpiece in the mouth, checking that there are no leaks and that the patient is not obstructing or bending it, ask him/her to: a) breathe in as much air as he/she can with a pause at the total lung capacity (TLC) for less than 1s28; b) blow hard and fast and c) prolong the following expiration without stopping until told to do so. In cases in which only the forced expiration is to be measured or there are no antibacterial filters, the patient should insert the mouthpiece after step (a) and try not to breathe from the tube. The technician must monitor the patient and watch the manoeuvre being performed. If defects are noted that could alter it, stop the manoeuvre so as not to tire the patient and correct them. If inspirometry is also to be performed, ask the patient to breathe in deeply until TLC without removing the mouthpiece from the mouth. In the case of children, using graphic incentives is useful for achieving better cooperation, especially in the time and volume of the manoeuvre, in addition to performing a test manoeuvre beforehand.29
To perform slow spirometry, tell the patient that they must: a) breathe calmly through the mouthpiece, at least 3 breaths to verify that the baseline (functional residual capacity [FRC]) is stable; b) breathe in until TLC, and c) blow slowly until the residual volume (RV). Alternatively, a slow expiration may be performed until RV, before the inspiration until TLC, which usually facilitates the manoeuvre in the case of air trapping. A nose clip should always be used in slow spirometry, to prevent possible air leaks on breathing through the nose. A minimum of 3 manoeuvres should be performed, separated by 1min.7
Acceptance CriteriaThe decision on whether a forced spirometry manoeuvre can be considered acceptable must take into account its start, course and completion.
- 1.
The start must be rapid, without hesitation. The main criterion for a proper start requires a back extrapolated volume (BEV) less than 0.15l or 5% of the FVC (0.08l or 12.5% FVC in pre-school children).29,30 As an additional criterion to assess the start of the manoeuvre, the time to reach the peak expiratory flow (PET) can be used; this must be less than 120ms2. If it is greater, tell the patient to blow faster at the start.
- 2.
The course of the expiratory manoeuvre should be continuous, without any artefacts or evidence of coughing in the first second that could affect the FEV1. To verify this, both the volume-time and the flow-volume graph should be monitored. If the course of the manoeuvre is not incorrect, generally due to coughing or excessive pressure and closure of the glottis, ask the patient to repeat it in a more relaxed manner (while continuing to blow hard) and not to reduce the force generated until the end of the expiration.
- 3.
The end of the manoeuvre must not show early or abrupt interruption of the expiration, so volume changes must be less than 0.025l for ≥1s. The final “plane” of the manoeuvre is only seen in the volume-time curve. The manoeuvre should last at least 6s. Young adults may have difficulty in maintaining the expiration for more than 4s, sometimes less. In these cases, verify that the end has not been abrupt. In children under 6 years of age, a duration of at least 1s should be attempted; between 6 and 8 years, it should be equal to or greater than 2s,30 and between 8 and 10 years, to 3s.29 In the case of a poor ending, ask the patient not to stop until told to do so, even though it seems that there is no output of air.
The equipment usually indicates if any of the errors detailed occurs. A manoeuvre will be considered useful (the spirometric parameters can be derived from it) when it has a good start and there are no artefacts in the first second. It will be considered acceptable (the errors must be taken into account to determine whether the spirometric parameters obtained can be used) when there are no errors at the start, during or at the end of the test.
Main Error SourcesThe circumstances that most often cause incorrect manoeuvres are:
- -
Lack of or incorrect calibration/verification, or environmental data.
- -
Poor patient preparation, with non-compliance with pharmacological and non-pharmacological recommendations.
- -
Poor instructions by the technician, before and during the manoeuvre.
- -
Early completion of the expiration (expiration time less than required, excessive final flow or abrupt end-of-test morphology); slow, hesitant start; presence of coughing or glottic closure during the manoeuvre; or air leakage during the forced expiration.
- -
Poor patient collaboration. If the patient does not improve, after advising them that good manoeuvres cannot be obtained without their cooperation, this should be indicated along with the results.
The difference between the best two acceptable VC, IC, FVC and FEV1 should be less than 0.15l. In patients with FVC less than 1l, a repeatability criterion <0.10l is recommended.9 In children, 2 manoeuvres will be considered repeatable when the difference in the FVC and FEV1 is <0.10l or <10%. A minimum of 3 acceptable manoeuvres should be carried out, with a maximum of 8, leaving sufficient time between them for the patient to recover their strength. In children, a minimum of 2 acceptable manoeuvres is considered sufficient, with no recommended maximum.
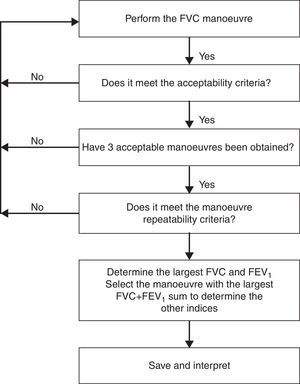
Analysis of the MeasurementsSelection of ResultsThe largest VC, IC, FVC and best FEV1 should be selected from all the acceptable manoeuvres with no artefacts, even if their values do not come from the same manoeuvre. The rest of the parameters should be obtained from an acceptable curve where the sum of the FVC and FEV1 values reach their maximum value (Fig. 1).
Flow diagram for the application of acceptability and repeatability criteria. Adapted from Miller et al.9
At present, almost all spirometers incorporate mathematical algorithms that allow the quality of the manoeuvre to be evaluated automatically, selecting the best manoeuvre performed. Despite their usefulness, it is recommended that the technician is the one who verifies that the selection is correct or selects the best results manually.
Indicators/Classification of Quality of MeasurementsThe use of a grading system has been proposed to assess the quality of the spirometry depending on the number of acceptable manoeuvres and the repeatability of the FEV1 and FVC (Table 5).9,31,32 Spirometries with grade A and B are considered good quality, and grade C of sufficient quality. Grade D spirometries and higher are not valid for interpretation. This quality classification has been shown to be useful in both epidemiological studies and in clinical practice, having been implemented automatically in some spirometers and referenced in many publications.2,23,31,33
Quality Grades for Forced Spirometry.
| Grade | Description |
| A | Three acceptable manoeuvres (without errors) and a difference less than or equal to 0.15l between the 2 best FVC and FEV1 |
| B | Three acceptable manoeuvres (without errors) and a difference less than or equal to 0.2l between the 2 best FVC and FEV1 |
| C | Two acceptable manoeuvres (without errors) and a difference less than or equal to 0.2l between the 2 best FVC and FEV1 |
| D | Two or 3 acceptable manoeuvres (without errors) and a difference less than or equal to 0.25l between the 2 best FVC and FEV1 |
| E | One acceptable manoeuvre (without errors) |
| F | No acceptable manoeuvres (without errors) |
Nevertheless, it should be taken into account that in around 10%–20% of cases, it is not possible to achieve good quality manoeuvres despite the technician's best efforts and good patient cooperation.31,33
Presentation of the ResultsTable 6 shows a proposal for presentation of the basic results of a forced spirometry, with a selection of the main variables and quality control data (Appendix 1). The choice of reference values and expression of the results require specific consideration.
Sample Table for Presentation of the Basic Results of a Forced Spirometry With a Bronchodilator Test.
| Parameter | Baseline | RV | LLN | %RV | Post | %RV | %Change |
| FVC, l | 4.87 | 4.93 | 4.06 | 99 | 4.93 | 100 | +1 |
| FEV1, la | 3.78 | 3.95 | 3.20 | 96 | 3.95 | 100 | +4.5 |
| FEV1/FVC | 0.78 | 0.8 | 0.71 | 0.8 | |||
| PEF, l/s | 8.3 | 9.0 | 7.55 | 92 | 8.5 | +3.5 | |
| FEF25%–75%, l/s | 3.65 | 3.78 | 2.12 | 97 | 3.78 | 100 | +3.5 |
| Quality control | Errorsb | Errorsb | |||||
| Quality grade | A | B |
FEF25%–75%, mid expiratory flow; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; LLN, lower limit of normal; PEF, peak expiratory flow; Post, post-bronchodilator; RV, reference value; %RV, percentage of reference value.
The parameters for lung function tests have large interindividual variability, and depend on the patients’ anthropometric characteristics (sex, age, height, weight and race). Spirometry interpretation is based on comparing the values produced by the patient with those that would theoretically correspond to a healthy individual with the same anthropometric characteristics. This theoretical or reference value is obtained from prediction equations.34
In general, it is advisable to use the set of prediction equations that best fits the particular area. Nevertheless, the most usual procedure is to select equations closest to the patient population (in our case the Spanish population); in this respect, the previous SEPAR guidelines recommended those of Roca et al.1,35,36 However these equations do not have sufficient subjects over 70 years of age and, therefore, introduce bias in their interpretation. Moreover, there is a large and growing population of patients eligible for spirometry over this age, or of non-European ancestry. García-Río et al.37 recently published equations for the 65–85 year range, which allow this age group to be properly covered.
These guidelines recommend using the reference values of Casan et al.35 for children (range 6–20 years), those of Roca et al.36 for adults (range 20–65 years) and those of García-Río et al.37 for elderly patients (range 65–85 years) (Table 7). These reference equations should only be used in Caucasian subjects.
Spirometric Reference Values Recommended in Spain.
| Author, Age range | Sex | Parameter | Equation | r | ESE |
| Casan (6–20 years)35 | M | FVC (l) | 0.02800 H+0.03451 W+0.05728 A–3.21 | 0.947 | 0.443 |
| FEV1 (l) | 0.02483 H+0.02266 W+0.07148 A–2.91 | 0.945 | 0.378 | ||
| PEF (ls−1) | 0.075 H+0.275 A–9.06 | 0.907 | 1.073 | ||
| FEV1/FVC (%) | |||||
| FEF25%–75% (ls−1) | 0.038 H+0.140 A–4.33 | 0.832 | 0.796 | ||
| F | FVC (l) | 0.03049 H+0.02220 W+0.03550 A–3.04 | 0.935 | 0.313 | |
| FEV1 (l) | 0.02483 H+0.02266 W+0.07148 A–2.91 | 0.945 | 0.378 | ||
| PEF (ls−1) | 0.073 H+0.134 A–7.57 | 0.879 | 0.831 | ||
| FEV1/FVC (%) | |||||
| FEF25%–75% (ls−1) | 0.046 H+0.051 A–4.30 | 0.789 | 0.651 | ||
| Roca (20–65 years)36 | M | FVC (l) | 0.0678 H–0.0147 A–6.0548 | 0.72 | 0.53 |
| FEV1 (l) | 0.0514 H–0.0216 A–3.9548 | 0.75 | 0.451 | ||
| PEF (ls−1) | 0.0945 H–0.0209 A–5.7732 | 0.47 | 1.47 | ||
| FEV1/FVC (%) | −0.1902 A+85.58 | 0.4 | 5.36 | ||
| FEF25%–75% (ls−1) | 0.0392 H–0.043 A–1.1574 | 0.55 | 1.0 | ||
| F | FVC (l) | 0.0454H–0.0211A–2.8253 | 0.75 | 0.403 | |
| FEV1 (l) | 0.0326H–0.0253A–1.2864 | 0.82 | 0.315 | ||
| PEF (ls−1) | 0.0448H–0.0304A+0.3496 | 0.47 | 1.04 | ||
| FEV1/FVC (%) | −0.244 A–0.1126 W+94.88 | 0.54 | 5.31 | ||
| FEF25%–75% (ls−1) | 0.023 H–0.0465 A–1.1055 | 0.70 | 0.68 | ||
| García-Río (65–85 years)37 | M | FVC (l) | 0.0001572 H2–0.00000268 A3+0.223 | 0.477a | 0.4458 |
| FEV1 (l) | 0.0001107 H2–0.0445 A+2.886 | 0.464a | 0.3797 | ||
| PEF (ls−1) | 0.07092 H–0.000939 A2+0.347 | 0.221a | 1.7378 | ||
| FEV1/FVC (%) | −00198 A2+87.472 | 0.083a | 5.2655 | ||
| FEF25%–75% (ls−1) | 0.02635 H–0.0604 A+2.042 | 0.219a | 0.7241 | ||
| F | FVC (l) | 0.0003171 H2–0.0351 A–6.368 BSA+0.05925 W+3.960 | 0.589a | 0.3046 | |
| FEV1 (l) | 0.0001726 H2–0.0326 A–2.303 BSA+0.000122W2+3.398 | 0.527a | 0.2741 | ||
| PEF (ls−1) | 0.0002283 H2–0.0644 A+4.001 | 0.209a | 1.1932 | ||
| FEV1/FVC (%) | −0.155 H–0.184 A+116.096 | 0.048a | 5.4974 | ||
| FEF25%–75% (ls−1) | 0.02030 H–0.0440 A+1.538 | 0.202a | 0.5828 |
BSA, body surface area (in m2); A, age (in years); F, female; FEF25%–75%, mid expiratory flow; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; M, male; W, weight (in kg); PEF, peak expiratory flow; H, height (in cm).
In 2012, new multiethnic reference equations were published for the 3 to 95 year age range, using 97 759 spirometries from 72 centres in 33 countries.38 This could have great future impact, both due to the number of subjects included in the equations and the wide age range, as well as the fact that they can be applied to different ethnicities. However, comparisons have not been made with the values traditionally used in Spain, although they undoubtedly raise great expectations for reaching international harmonisation.
Expression of ResultsFixed Percentage of the Predicted ValueThe fixed value of 80% of the predicted value as a limit of normal does not have any statistical basis. Although 80% of the predicted is close to the 5th percentile in subjects of average age and height, in older or short subjects, this fixed value can classify them erroneously as “abnormal”, while young, tall subjects may be erroneously classified as “normal”.34 For this reason, the lower limit of normal should be added to the fixed percentage value.
Lower Limit of NormalFor spirometry, values below the 5th percentile are considered lower than the expected range (below the lower limit of normal [LLN]). If they are not included in the prediction equations provided by the spirometers, the percentiles can be calculated using the estimated standard error (ESE) of the equation. The LLN is equal to the predicted value minus (1.645×ESE). Thus, for each parameter (FVC, FEV1, FEV1/FVC, PEF and FEF25%–75%), and depending on the sex, age and height, a LLN will be obtained.
Finally, it has been proposed that it would be more correct to report results using the “z score” (z=(x−μ/σ)), where x is the value obtained, μ is the population mean for the subject's anthropometric characteristics and σ is the standard deviation.39,40 The “z score” allows abnormalities and functional changes to be interpreted with a number that is proportional to σ (2σ is equivalent to the 5th percentile).39,40
InterpretationSpirometry is useful for the diagnosis, severity assessment and monitoring of the progression of airflow limitation. Its interpretation should be clear, concise and informative, and its evaluation should be individualised, taking into account the graph and numerical values.34
The spirometry is considered normal when its values are higher than the lower limit of the confidence interval (LLN). The LLN is around 80% of the theoretical value of the FEV1, FVC and VC, 0.7 for the FEV1/FVC ratio, and approximately 60% for the FEF25%–75% in subjects under 65 years and who are neither very tall nor very short. However, these values are only approximations, so the LLN determined from the reference equations should be used.
Airflow limitation is defined by a low FEV1/FVC ratio (below the LLN).41,42 In clinical practice, its use has set the definition of obstruction as an FEV1/FVC ratio less than 0.7,43 due to its simplicity, although this criterion is less precise and gives rise to false negatives in young patients and false positives in the elderly.44,45
Airflow limitation causes a disproportionate decrease in the flow rates at low volumes, which is reflected in the concave shape of the flow-volume curve, and quantitatively is manifested in a proportionally greater reduction in the FEF75% or FEF25%–75% than the FEV1.46
An unusual circumstance is when the FEV1 and FVC decrease concomitantly, and the FEV1/FVC is normal or almost normal. This pattern may reflect the patient's inability to inhale or exhale fully, or small airways collapse in the initial phases of the expiration. In this situation, it may be useful to replace the FVC with the slow VC, and to calculate the Tiffeneau ratio (FEV1/VC), which in this defect is below the LLN.6,47 The severity of airflow limitation is classified according to the FEV1 value, following ATS/ERS recommendations34 (Table 8), although there are also disease-specific classifications, such as the one proposed by the GOLD guidelines.43 These severity cut-off points are arbitrary.48,49
Classification of the Severity of Airflow Limitation.
| Level of severity | FEV1, % reference value |
| Mild | >70% |
| Moderate | 60%–69% |
| Moderately severe | 50%–59% |
| Severe | 35%–49% |
| Very severe | <35% |
Taken from Pellegrino et al.34
“Non-obstructive” airflow limitation is defined as a low FVC with a FEV1/FVC ratio above the LLN, or even the mean reference value. A restrictive disorder should be suspected when the FVC is below the LLN, the FEV1/FVC ratio exceeds its LLN and the flow-volume curve has a convex morphology. However, this can only be confirmed if a reduction in the TLC is detected (<5th percentile of the reference value).50
The co-existence of an obstructive and non-obstructive defect in a patient is defined when both the FVC and the FEV1/FVC ratio are below their respective LLNs. To determine whether the origin is air trapping (hyperinflation) or genuine restriction, the TLC should be measured.51,52 These guidelines generally recommend confirming the presence of restriction when the FVC or VC is low by measuring the TLC.53
It is often difficult to determine when a change in a variable over a period of time reflects a real change in lung function or is simply due to the variability of the test. The significance of the changes varies for each parameter, time period and according to the type of patient (Table 9). Measurements for follow-up of a disease should be made in a stable phase, although in some entities such as asthma, it may be appropriate to evaluate lung function during exacerbations. The change is more likely to be real when it occurs in more than one variable, the larger it is and when it is accompanied by changes in the patient's symptoms.54,55
Significant Changes in the Spirometric Parameters Over Time (%).
| FVC | FEV1 | FEF25%–75% | |
| Day-to-day | |||
| Normal subjects | ≥5 | ≥5 | ≥13 |
| COPD patients | ≥11 | ≥13 | ≥23 |
| Week-to-week | |||
| Normal subjects | ≥11 | ≥12 | ≥21 |
| COPD patients | ≥20 | ≥20 | ≥30 |
| Year-to-year | ≥15 | ≥15 | |
Taken from Pellegrino et al.34
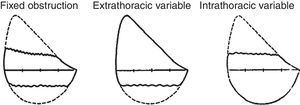
Diagnostic evaluation of upper airway obstructions using spirometry has now been pushed into the background by imaging and endoscopic techniques. These disorders sometimes do not reduce either the FEV1 or the FVC, although they do cause significant changes in the PEF. A FEV1/PEF ratio >8 indicates the presence of upper airway stenosis, once an initial insufficient effort has been ruled out. The observation of a plateau in the forced inspiratory flow, with or without a plateau in the expiratory flow, suggests variable extrathoracic obstruction of the upper airways. A plateau in the expiration without inspiratory impairment suggests a variable intrathoracic obstruction. The presence of a plateau in both the inspiratory and expiratory loop suggests the existence of fixed stenosis of the upper airways.56 The impact of these lesions on the maximal flow rates will depend on the location of the obstruction, the type (fixed or variable) and the anatomical extension (Table 10) (Fig. 2).
Parameters of the Flow-Volume Curve Recommended for Differentiating Upper Airways Obstructions.34
| Extrathoracic obstruction | Intrathoracic obstruction | ||
| Fixed | Variable | ||
| PEF | Decreased | Normal or decreased | Decreased |
| FIF50% | Decreased | Decreased | Normal or decreased |
| FIF50%/FEF50% | ∼1 | <1 | >1 |
FEF50%, forced expiratory flow when 50% of the vital capacity has been exhaled; FIF50%, forced inspiratory flow when 50% of the vital capacity has been inhaled; PEF, peak expiratory flow.
Taken from Pellegrino et al.34
The test for reversibility of airflow limitation, commonly called the bronchodilation test, consists of measuring the lung function before and after administering a fast-acting bronchodilator. Although the test has diagnostic, prognostic and therapeutic usefulness, the assumption that the response to a single bronchodilation test is sufficient to determine the reversibility and therapeutic benefits of the drugs is erroneously simplistic, as the same individual may have a different response at different times and depending on the bronchodilator.49,57-62
The bronchial reversibility study is indicated when asthma is suspected, and an obstructive spirometry is obtained for the first time,34,50,51,63 to evaluate a possible additional response or alternative treatment regimens in patients with known reversibility whose FEV1 remains below 80% of the predicted (or known baseline value) despite treatment,64 to determine the degree of disability65 and for preoperative evaluation when there is airflow limitation.66
In addition to the contraindications for spirometry (Table 2), it should not be performed when there are known or probable adverse reactions to the bronchodilator to be used.67
Administration of the BronchodilatorThe type of bronchodilator, dose and time of measuring the effect influence the response obtained. The use of short-acting β2-adrenergic agonists (SABA) or ipratropium bromide9 is recommended, although the onset of action of formoterol is also sufficiently rapid to be used.68 Although there is a small group of asthmatic patients who respond better to ipratropium,69,70 the bronchodilator effect of SABA is usually greater than the effect of ipratropium in these patients.69-71 However, in many patients diagnosed with COPD, ipratropium bromide is more effective,69,72 and the combination of a SABA and ipratropium bromide is even more so.73,74
Except in some circumstances, such as known arrhythmias, tremor or previous adverse reaction, where it is preferable to administer lower doses, the administration of 400μg of salbutamol in 4 puffs (100μg per puff) separated by 30s intervals is recommended. If ipratropium bromide is used, a total dose of 160μg (8×20μg) should be given. In both cases, it is preferable to use pressurised cartridges with a spacer device, following the correct technique. The administration of terbutaline, both in a pressurised cartridge and as a dry power, may be an alternative to salbutamol. The use of high doses, such as those mentioned, almost always ensures that the bronchodilator effect is close to the maximum area of the dose-response curve, thus minimising the variability due to the distribution of aerosol and the patient's technique.9,75,76
Fifteen minutes after the inhalation of salbutamol, or 30min after the inhalation of ipratropium bromide, a second series of spirometric manoeuvres should be performed, following the same quality and repeatability criteria as stated above.9 The administration of both drugs, measuring the response at 30min, increases the proportion of patients with COPD who have a positive bronchodilator test.77 There is no evidence that indicates the sequence in which both drugs should be administered, or if they should be given consecutively or separated by an interval.69
The bronchodilator can be administered by any of the usual means (nebuliser, pressurised cartridge, dry power inhaler). Each laboratory must standardise the procedure and know the system characteristics and drug deposition in the airways. These guidelines recommend performing the bronchodilator test with a short-acting β2-adrenergic agonist, at doses equivalent to 400μg of salbutamol in a pressurised cartridge with a spacer device.19 The administration of a short-acting β2-adrenergic agonist and ipratropium bromide could increase the sensitivity of the test, particularly in patients with COPD.73,73,77 If so, this must be noted in the report.
Analysis of the ResponseThe 3 most common indices for evaluating the bronchodilator response are the percentage change over the baseline value,78–80 the percentage change over the predicted value30,74 and the absolute change.79,80 An improvement in the FEV1 or in the FVC ≥12% and ≥0.2l is recommended as a reversibility criterion,63,81 due to its greater sensitivity. The expression of the change as a percentage of the predicted value normalises the result for gender, initial FEV1 and age, and eliminates the mathematical bias (the lower the baseline FEV1, the greater the percentage response),23,58,82 but it is less sensitive,49,59,83 especially in patients with more impaired function.59 In patients with emphysema, the FVC has been shown to be more sensitive than the FEV1,84,85 and its response appears to be better correlated with the clinical changes and tolerance to effort in patients with hyperinflation.84–88
The use of the FEF25%-75% or instantaneous flows is not recommended for assessing reversibility. Although it has been reported than an increase of 20% in the PEF may be indicative of reversibility,89,90 its use is not recommended either for the assessment of reversibility in lung function laboratories, or when the reversibility test is performed for diagnostic purposes.
In many patients with airflow limitation, in whom the FEV1 or FVC does not improve significantly, a significant increase in the IC has been observed however,75,88,91,92 and shows a better relationship with the clinical improvement and the effort capacity than changes in the FEV1.86–88,93,94 Therefore, an increase in the IC of 10% with respect to the previous value has also been proposed as a reversibility criterion.75 Nevertheless, this significant response criterion is not sufficiently proven to be recommended for general use.
Usefulness and LimitationsReversibility is a characteristic of asthma, and therefore the reversibility test is useful and is indicated in all patients with suspected asthma.63,89,95,96 However, the absence of significant reversibility does not permit the diagnosis of asthma to be ruled out.63,89,95,96
The response to bronchodilators cannot differentiate between COPD and asthma,43,97 although improvements of more than 0.4 l suggest asthma,97 or at least a mixed phenotype.98 One concept that has changed in recent years, in the wake of large clinical trials, is that the presence or absence of bronchodilation does not appear to predict symptomatic relief, changes in exercise capacity or long-term response to corticosteroids or bronchodilators with sufficient accuracy,59,99 so the bronchodilator test does not have any value as a treatment guide. It has been found that the reversibility is associated with an accelerated decline in the FEV1,100 but not all studies confirm this association.
Special SituationsOffice SpirometersThere are spirometers currently on the market that can be used in both the specialised care and “office” setting.2,101–104 Their main advantages lie in the lower cost and smaller size, although some of their characteristics can present limitations for their widespread use.105–107 The general requirements for this equipment are as follows2,108,109:
- 1.
Office spirometers “may” only provide FEV1, FEV6and FEV1/FEV6ratio values. The use of FEV6 has several advantages16,110: a) it is easier for the patient and technician when the manoeuvres only last 6s; b) it avoids technical problems with flow sensors related with precision, as it has to measure very low flows; c) the FEV6 is more repeatable than the FVC in patients with airway obstruction, and d) using the FEV6 reduces the total time for performing the test. One limitation, however, is the existence of fewer reference values than for the FVC and the possibility of underestimating the patient's real capacity.45 The existence of obstruction is identified when the FEV1/FEV6 and FEV1 are below their LLN.
- 2.
Quality standards in the manoeuvre. Many of the basic standards for assessing the reliability of the signals2,111,112 are already included in these devices. It is important that additional checks can be made to evaluate the acceptability of the manoeuvres in spirometers that do not have curve printing or display. The spirometer should show the quality control criteria, which should be monitored electronically, together with messages when errors are detected. The devices must present the spirometry results in numerical values, and they should only be interpreted if all the manoeuvres meet the quality criteria.
- 3.
The existence of screens and graphs of the flow-volume curve may be optional. Graphic expression of the spirometry implies the possibility that the technicians who perform the test, as well as the physicians who interpret the results, can recognise if there are problems with respect to the quality of the test.113 However, a graphics screen increases the size, cost and complexity of spirometers, reducing their acceptance in the primary care setting. Most office devices do not show the flow-volume curve or the volume-time curve during the manoeuvre.107
- 4.
Materials explaining the operation of office spirometers should be easy to understand. These materials should include standard operating procedures, audiovisual training aids (such as a video tape or multimedia CD-ROM), documents describing the potential risks and benefits of the spirometry, as well as the interpretation of results and limitations of the test.
- 5.
There should be economic solutions that can replace the daily use of 3 l calibration syringes.2,109 Biological controls are recommended and in this case, the range of repeated measurements of FEV1 and FEV6 every 10 days should not exceed 10% of the mean. Most manufacturers state that their portable devices do not have to be calibrated before performing the measurements. Although this is a potential advantage, it is reasonable to say that periodic calibration or checking of portable spirometers, daily if possible, is vitally important for demonstrating the stability of the measurements.
- 6.
The parameters should be corrected to BTPS conditions. The device should detect the temperature automatically, making an automatic correction to BTPS units (Appendix 2).
- 7.
Hygiene measures. Personnel performing spirometries should be instructed in correct hand-washing techniques before and after attending each patient. The use of disposable mouthpieces is sufficient to minimise the risk of transmission of infections. The use of filters fitted to the mouthpiece is not mandatory, provided that the patient only performs the forced manoeuvre and does not inhale from the apparatus.
In short, office spirometer manufacturers should focus particularly on improving the precision of FVC measurements, calibration problems and the ability to show flow-volume and volume-time curves, in order to evaluate the quality of the manoeuvres. Although the software in portable spirometers allows the expression of volumes and flow measured as a percentage of the predicted value, it is essential that the user has the possibility of using reference values that correspond to the characteristics of the patient population studied (Table 11).
Conditions for the Interpretation of Office Forced Spirometry Results.
| 1. The quality of the test must be correct |
| 2. Proper reference values should be used to calculate the predicted values and the LLNs of the FEV1, FEV6 and FEV1/FEV6 (they should be provided by the equipment) |
| 3. If the FEV1/FEV6 and FEV1 are below the LLN in a good quality test, the existence of airflow limitation is accepted |
| 4. The FEV1 should be reported as a percentage of the patient's predicted value. Optionally, the severity of the obstruction can be classified |
| 5. The acceptability of the manoeuvre should be automated and should show repeatability messages |
In addition to maximal expiratory flow metres,114 the development of pocket spirometers provides systems that incorporate measurements such as the FEV1 and FEV6.115 Their use is recommended for home monitoring of various diseases, such as asthma, but not as a diagnostic method. The advantage of these spirometers lies in that these are simple, safe and portable devices that are normally cheap. However, compared with standard spirometry, they are relatively insensitive for detecting mild obstruction and measure parameters that are very dependent on the effort, with greater variability, which, in the case of an abnormal study, requires these findings to be confirmed using a conventional test.
Integration in Electronic Record SystemsIn recent years, information and communication technologies applied to the healthcare setting have undergone major development. The new systems, usually installed in both primary care centres and in hospitals, store patient prescriptions in a single point and allow the exchange of information. According to Spanish law, it is illegal to have databases on equipment that do not comply with Spanish Organic Law 15/1999, of 13 December, on Protection of Personal Data. This issue must be raised with managers and manufacturers before acquiring new equipment. In the healthcare setting, in order to facilitate the integration of the applications, various standards have been developed, such as XML, DICOM or HL7.116 These standards were born from the need to link the various peripheral healthcare systems, which are increasingly computerised, with a single central system that allows a patient's common record to be held, thus obtaining better flow of information and more security throughout the process.
There is a growing demand for integrating the information obtained in the spirometry in documents that use intraoperative formats (such as HL7 or CDA R2) to have access to the patient's spirometry history through the electronic medical record. The definition of these standards is essential for ensuring that they are adopted by spirometer manufacturers.117 Using this process, bases are set up to facilitate access to the spirometry from all the care settings, and in turn it is a fundamental technical element for designing quality control programmes for the examinations.118
The use of these technologies enables the reports to be shared via information systems, and also allows the physician to see the spirometry digitally from his or her work station, and to access the test history for each patient. To complete this process, office spirometers must also have the ability to send the data obtained, to operate not as an isolated element, but integrated in a common operating system. Once a spirometer has a digital information output, its form of integration can vary, with different studies carried out in recent years (web-based system, TechEd's network, telephone support or discs).119-122
Conflict of InterestsThe authors declare that they have no conflict of interests.
| ID (patient identification) |
| Name of patient |
| Type of data (SP followed by E5expiratory or I5inspiratory, followed by S5only or B5best curve) |
| Atmospheric pressure (mmHg) |
| Temperature (°C) used in the conversion to BTPS units |
| Relative humidity (%) |
| Quality grade for FVC (A, B, C, D, E or F) |
| Quality grade for FEV1 (A, B, C, D, E or F) |
| Interpretation code (according to ATS interpretation scheme) |
| Manoeuvre deleted (Y or N) |
| Manoeuvre acceptable (Y or N) |
| Quality control code according to technique (A, B, C, D, E or F) |
| Automated quality control code (A, B, C, D, E or F) |
| Plateau reached (Y or N) |
| Reviewed (Y or R for “needs reviewed” or “reviewed”) |
| Date of review (DD/MM/YYYY) |
| Reviewer's initials |
| BTPS factor (x.xxx) |
| Spirometer manufacturer |
| Spirometer model |
| Spirometer serial number |
| Type of spirometer |
| Name of supplier |
| Town/city |
| State/region |
| Postcode |
| Country |
| Telephone number |
| Date of calibration (DD/MM/YYYY) |
| Time of calibration (HH:MM) |
| Calibration result (C or F for “correct” or “failed”) |
| Date (DD/MM/YYYY) |
| Time (HH:MM) |
| Technician's ID (technician's initials or identification code) |
| Manoeuvre number |
| Age (whole years) |
| Height (cm) |
| Weight (kg) |
| Sex (M or F) |
| Race (2 character code) |
| Date of birth (DD/MM/YYYY) |
| Source of reference values (surname of first author and date of publication; for example, “Knudson 1983”) |
| Correction factor for reference values (x.xx, 1.00 for no correction) |
| Position during the test (decubitus, seated or supine) |
| Type of test (pre-, post-, bronchodilator, methacholine concentration or dose) |
| FVC (x.xx l) |
| Extrapolated volume (x.xx l) |
| FEV1 (x.xx l) |
| FEV6 (x.xx l) |
| PEF (xx.xx ls−1) |
| FEF25%-75% (xx.xx ls−1) |
| VC (x.xx l) |
| Forced expiration time (s) |
| Time to reach PEF (s) |
| FVC predicted (x.xx l) |
| FEV1 predicted (x.xx l) |
| FEV6 predicted (x.xx l) |
| FEV1/FVC predicted (x.xx l) |
| FEV1/FEV6 predicted (x.xx l) |
| FEF25%–75% predicted (xx.xx ls−1) |
| LLN for FVC (x.xxl) |
| LLN for FEV1 (x.xx l) |
| LLN for FEV6 (x.xx l) |
| LLN for FEV1/FVC (x.xx l) |
| LLN for FEV1/FEV6 (x.xx l) |
| LLN for FEF25%-75% (xx.xx ls−1) |
| FEF25% (xx.xx ls−1) |
| FEF50% (xx.xx ls−1) |
| FEF75% (xx.xx ls−1) |
| FEF90% (xx.xx ls−1) |
The results obtained in the spirometry are measured in ambient temperature and pressure conditions (ATPS). However, these conditions are different to those within the lungs, where the air is at a temperature of 37°C and the water vapour saturation is 100% (which corresponds to 47mmHg), conditions which are called BTPS (body temperature [BT] and pressure saturated with water vapour [PS]). Since the volume of a gas varies in relation to the temperature and pressure, to avoid underestimation in the measurements, a correction factor should be applied which converts the values obtained (ATPS) to BTPS conditions. The correction should be applied to both the flow rates and the volume, and will depend on the type of pneumotachograph and placement of a filter. Most current spirometers have automatic temperature measurement with good precision (±1%), and in some cases also record the atmospheric pressure and relative humidity, which permits the correction to BTPS conditions to be done automatically.
If the spirometer does not perform the correction to BTPS automatically, it should offer the possibility of entering the temperature and pressure data manually. In general, the temperature range varies between 17 and 37°C and the atmospheric pressure between 440 and 775mmHg. If there are major temperature variations in the same session, the most frequent corrections to BTPS conditions will be performed. It is recommendable that spirometer manufacturers specify the temperature and pressure ranges in which each device can be used. In this respect, standard ISO 26782; 2009 establishes that for the validation of a spirometer, it should be subjected to tests with different pressure and humidity levels, mimicking the different situations in which it may be used.