Anaemia is one of the extrapulmonary manifestations of chronic obstructive pulmonary disease (COPD). Its real prevalence, physiopathology and clinical repercussion are unknown. The objectives of our study were: to determine the prevalence of anaemia in patients with stable COPD not attributable to other causes and to establish the relationship of anaemia with clinical, prognostic and inflammatory markers with an important role in COPD.
MethodsThe study included stable COPD patients with no other known causes of anaemia. The following tests were carried out: respiratory function tests; serum determination of erythropoietin and inflammatory markers: high sensitivity C-reactive protein (hs-CRP), fibrinogen, interleukin 6 (IL-6), interleukin 8 (IL-8) and tumour necrosis factor α (TNF-α). Body mass index (BMI), Charlson and BODE indices, the number of exacerbations in the previous year, dyspnoea and quality of life were also calculated.
ResultsOne hundred and thirty patients were included. Anaemia prevalence was 6.2%. Mean haemoglobin value in anaemic patients was 11.9±0.95g/dL. Patients with anaemia had a lower BMI (P=.03), higher Charlson index (P=.002), more elevated erythropoietin levels (P=.016), a tendency to present a lower FEV1% value (P=.08) and significantly lower IL-6 values when compared to non-anaemic patients (P=.003).
ConclusionsIn our series, the anaemia associated with COPD was less prevalent than that published in the literature to date, and was related to certain clinical and inflammatory markers.
La anaemia es una de las manifestaciones extrapulmonares de la enfermedad pulmonar obstructiva crónica (EPOC). Su prevalencia, su fisiopatología y su repercusión clínica son desconocidas. Los objetivos de nuestro estudio son determinar la prevalencia de la anaemia en pacientes con EPOC en fase estable no atribuible a otras causas y establecer la relación de la anaemia con variables clínicas, pronósticas y marcadores inflamatorios con un papel relevante en la EPOC.
MétodosSe incluyeron pacientes con EPOC en fase estable sin otras causas conocidas de anaemia. Se realizaron pruebas de función respiratoria, determinación de eritropoyetina y marcadores inflamatorios séricos: PCR ultrasensible (PCR), fibrinógeno, interleucina 6 (IL-6), interleucina 8 (IL-8) y factor de necrosis tumoral alfa (TNF-α). Se registró el índice de masa corporal (IMC), el índice de Charlson y el BODE, el número de exacerbaciones en el año previo, la escala de disnea y la calidad de vida.
ResultadosSe incluyeron 130pacientes. La prevalencia de anaemia fue del 6,2%. El valour de hemoglobina en los pacientes con anaemia fue de 11,9±0,95g/dl. Los pacientes con anaemia tenían un IMC más bajo (p=0,03), un índice de Charlson mayor (p=0,002), niveles de eritropoyetina más elevados (p=0,016), una tendencia a presentar niveles más bajos de FEV1% (p=0,08) y valores significativamente más bajos de IL-6 (p=0,003) cuando se comparan con los pacientes no anémicos.
ConclusionesEn nuestra serie, la anaemia asociada a la EPOC es menos prevalente de lo publicado hasta la actualidad y guarda relación con determinados factores clínicos y marcadores inflamatorios.
Chronic obstructive pulmonary disease (COPD) is fundamentally characterised by the presence of airflow limitation that is not fully reversible.1 Great importance has recently been given to the role of inflammation in this disease, and may explain some of the extrapulmonary manifestations of COPD described in recent years,2–4 such as weight loss, muscle atrophy, increase in cardiovascular morbidity and mortality, altered bone metabolism, anaemia and depression.
The prevalence of anaemia in COPD varies widely according the study, and ranges between 4.9% and 33%.5–12 This disparity in the data could be due to various factors, such as the retrospective nature of some studies, use of heterogeneous populations (COPD in a stable or exacerbated phase; outpatients or inpatients), the use of different cut-off points to define anaemia and the existence of confounding factors such as the presence of other known causes of anaemia, like heart failure, renal failure, neoplasms, etc.
Therefore, we designed an epidemiological, observational, prospective study that would allow us, on one side, to determine the prevalence of anaemia not attributable to other causes in a sample of our patients with stable COPD, and on the other, to analyse the relationship of the anaemia with clinical and prognostic variables and inflammatory markers with a relevant role in COPD.
MethodsPatients diagnosed with COPD according to GOLD criteria,1 followed-up in Respiratory Medicine outpatients, and who had not presented acute exacerbations in the previous 2 months were enrolled. The study took place between July 2008 and June 2010.
In order to avoid possible selection bias, all patients diagnosed with bronchial asthma and those who had any disease or comorbidity that could affect haemoglobin levels and/or inflammatory markers were excluded (patients with thyroid disease, history of cancer in the last 5 years, liver disease, chronic heart failure, chronic renal failure, chronic inflammatory rheumatic disease, previous history of gastrointestinal bleeding or blood loss, vitamin B12 and folic acid deficiency).
The study was approved by the hospital Ethics Committee. All patients signed an informed consent before undergoing any study procedure. The patient's medical record was reviewed and they had a personal interview to confirm the absence of exclusion criteria.
The following epidemiological data were recorded: weight, height, body mass index (BMI), grade of dyspnoea according to the modified Medical Research Council (mMRC) scale, quality of life measured using the EQ-5D,13 history of smoking, usual pharmacological treatment, comorbidity measured using the Charlson index and number of moderate and severe exacerbations in the previous 12 months. All patients underwent forced spirometry with a bronchodilator test (Masterlab Screen Body Jaeger), 6-minute walk test and baseline arterial blood gases, and the BODE index was calculated (body mass index [B], airflow obstruction measured using the forced expiratory volume in one second (FEV1) [O], dyspnoea measured using the mMRC scale [D] and exercise capacity measured using the 6-minute walk test [E]).14
A peripheral venous blood sample was obtained for analysis of complete blood count, fibrinogen, serum biochemistry and erythropoietin (immunometric assay with chemiluminescent detection: Immulite 2000, Siemens). One of the peripheral blood samples was centrifuged and aliquoted before freezing at −70°C for subsequent analysis of inflammatory markers (IL-6, IL-8, TNF-α, high-sensitivity C-reactive protein [CRP]). Interleukins and TNF-α were analysed using an enzyme immunoassay technique (DRG diagnostic, GmbH, Germany) with detection limits of 2pg/mL for IL-6, 1.1pg/mL for IL-8 and 0.7pg/mL for TNF-α. High-sensitivity CRP was analysed by automated nephelometry (BNprospect, Siemens).
Patients who were included initially in the study but who subsequently presented abnormal thyroid hormone values (either high or low), vitamin B12 and folic acid deficiency, glomerular filtration rate (GFR) below 60mL/min15 and increased liver enzymes twice the upper limit of normal were excluded from the final analysis.
Anaemia was defined (according to WHO criteria) as the presence of haemoglobin levels <13g/dL in males and <12g/dL in females.16
Sample SizeThe following data were taken into account for calculating the sample size: 80% of patients with COPD and anaemia have high inflammatory markers and 20% of patients with COPD without anaemia also have them. Therefore, 17 patients would have to be included in each group for a 95% confidence interval and a statistical power of 90%. Considering recent publications that estimate that the prevalence of anaemia in stable COPD is 13%,5 a total of 130 patients would have to be included in the study to reach this number of patients in the COPD and anaemia group.
Statistical AnalysisEquality of means in the variables between patients with and without anaemia was tested using bootstrap analysis, since the samples from individuals with anaemia have an n of between 6 and 8 individuals and, therefore, it is not correct to compare and/or assume the hypothesis of normality. This method was therefore selected, as it does not require the assumption of certain patterns of behaviour. The maximum number of regenerated non-repeat samples is not arbitrary but depends on the sample size.17,18 In this study, it varied from 300 to 5000 replicates, depending on the number of patients.
In order to study the correlation between the variables, we used the Kendall Tau-b and Spearman Rho coefficients, non-parametric measures based on ranges, which allow bivariate relationships to be compared.19 A P value<.05 was considered significant.
Statistical analysis was performed using SPSS version 19 and, for the bootstrap, @RISK 4.5.
ResultsOne hundred and forty-seven patients diagnosed with COPD and under follow-up in outpatients were initially included, of whom 17 were excluded from the final analysis because they presented some of the previously described exclusion criteria. The most common cause of exclusion was renal failure in 6 cases (35%), followed by absence of COPD diagnosis (5 cases, 29.4%), abnormal thyroid profile (2 cases) and abnormal folic acid/vitamin B12 levels (2 cases: one due to abnormal liver profile and the other due to chronic heart failure). The prevalence of anaemia in COPD patients who were excluded (12) was 16.6%.
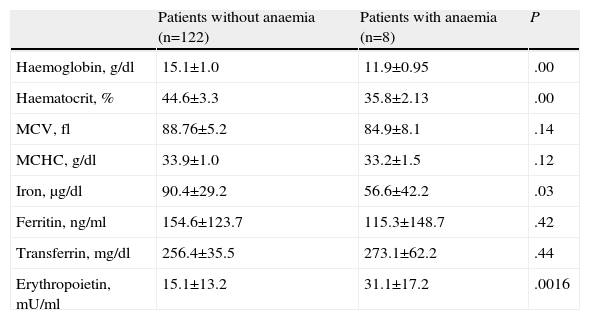
Of the 130 patients finally included in the study, 8 had anaemia, which represents a prevalence of 6.2%. In all cases, it was defined as normochromic normocytic anaemia with a high coefficient of variation in the mean cell volume, with low iron and normal transferrin and ferritin levels. These data are consistent with anaemia of chronic disease.
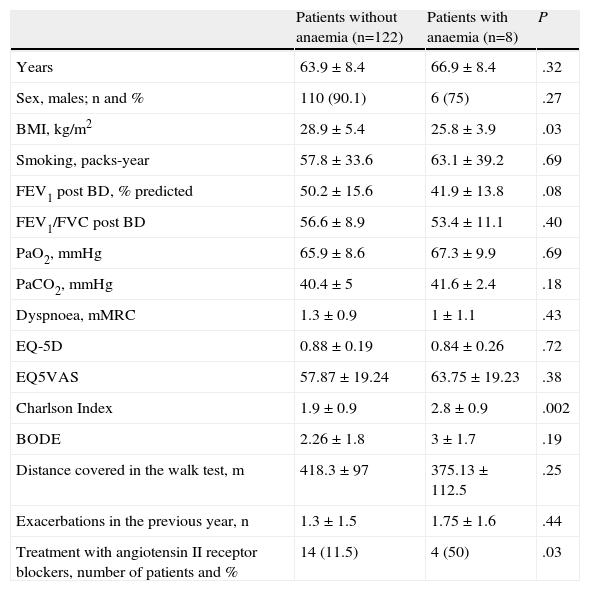
The anaemia was mild in most cases, with a mean haemoglobin value of 11.9±0.95g/dL. The main characteristics of patients in our series and the differences in the epidemiological characteristics and analytical data between the group of cases with and without anaemia are shown in Tables 1 and 2.
General Characteristics of Patients Included in the Study. Differences Between Groups.
| Patients without anaemia (n=122) | Patients with anaemia (n=8) | P | |
| Years | 63.9±8.4 | 66.9±8.4 | .32 |
| Sex, males; n and % | 110 (90.1) | 6 (75) | .27 |
| BMI, kg/m2 | 28.9±5.4 | 25.8±3.9 | .03 |
| Smoking, packs-year | 57.8±33.6 | 63.1±39.2 | .69 |
| FEV1 post BD, % predicted | 50.2±15.6 | 41.9±13.8 | .08 |
| FEV1/FVC post BD | 56.6±8.9 | 53.4±11.1 | .40 |
| PaO2, mmHg | 65.9±8.6 | 67.3±9.9 | .69 |
| PaCO2, mmHg | 40.4±5 | 41.6±2.4 | .18 |
| Dyspnoea, mMRC | 1.3±0.9 | 1±1.1 | .43 |
| EQ-5D | 0.88±0.19 | 0.84±0.26 | .72 |
| EQ5VAS | 57.87±19.24 | 63.75±19.23 | .38 |
| Charlson Index | 1.9±0.9 | 2.8±0.9 | .002 |
| BODE | 2.26±1.8 | 3±1.7 | .19 |
| Distance covered in the walk test, m | 418.3±97 | 375.13±112.5 | .25 |
| Exacerbations in the previous year, n | 1.3±1.5 | 1.75±1.6 | .44 |
| Treatment with angiotensin II receptor blockers, number of patients and % | 14 (11.5) | 4 (50) | .03 |
The values are shown in mean±standard deviation (SD), except for gender and treatment with angiotensin II receptor blockers, which are expressed as number of patients and percentage.
Characteristics of Analytical Parameters. Differences Between Groups.
| Patients without anaemia (n=122) | Patients with anaemia (n=8) | P | |
| Haemoglobin, g/dl | 15.1±1.0 | 11.9±0.95 | .00 |
| Haematocrit, % | 44.6±3.3 | 35.8±2.13 | .00 |
| MCV, fl | 88.76±5.2 | 84.9±8.1 | .14 |
| MCHC, g/dl | 33.9±1.0 | 33.2±1.5 | .12 |
| Iron, μg/dl | 90.4±29.2 | 56.6±42.2 | .03 |
| Ferritin, ng/ml | 154.6±123.7 | 115.3±148.7 | .42 |
| Transferrin, mg/dl | 256.4±35.5 | 273.1±62.2 | .44 |
| Erythropoietin, mU/ml | 15.1±13.2 | 31.1±17.2 | .0016 |
MCHC, mean cell haemoglobin concentration; MCV, mean cell volume.
Values are shown as mean±standard deviation (SD).
Patients in the anaemia group had a lower BMI (P=.03), higher Charlson index (P=.002) and higher erythropoietin levels (P=.016). Patients with anaemia had more severe COPD in terms of the post-bronchodilator FEV1%, although this did not reach statistical significance (P=.08). There were no differences in the age, sex, smoking history, dyspnoea, quality of life (measured using the EQ-5D), arterial PaO2 or PaCO2 values, exacerbations in the previous year, score in the BODE index or metres covered in the 6-minute walk test between patients with and without anaemia.
The haemoglobin levels showed an inverse correlation with the Charlson index (Kendall's Tau-b=0.13; P=.046, and Spearman's Rho=0.17; P=.044) and with the erythropoietin levels (Kendall's Tau-b=−0.15; P=.020, and Spearman's Rho=−0.21; P=.022). The correlations with the other parameters analysed, including the PaO2, PaCO2, FEV1%, distance covered in the 6-minute walk test, cumulative smoking (pack-years) or BODE index were not significant.
The usual treatment of patients in each group was recorded. Four patients with anaemia were on treatment with angiotensin II receptor blockers compared to 14 patients in the group without anaemia (50% vs 11.5%; P=.03), and one patient with anaemia was on treatment with angiotensin-converting-enzyme inhibitors compared to 21 in the group without anaemia (12.5% vs 17.2%; P=.61). The prevalence of anaemia in COPD patients not treated with these drugs was 3.3%. Only 6 of the 130 patients were on theophylline treatment, none of whom had anaemia.
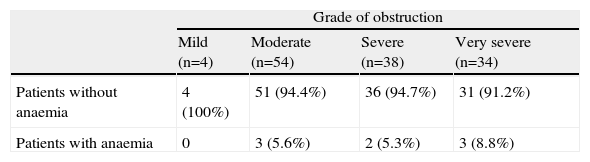
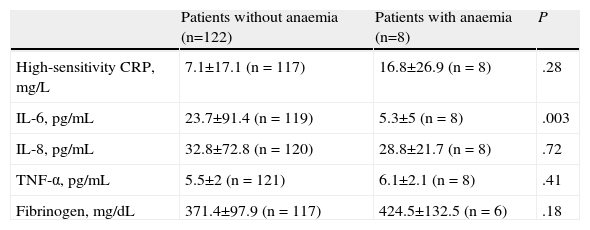
Table 3 shows the patient distribution according to the degree of obstruction: 8.8% of patients with very severe COPD had anaemia compared to 0% in patients with mild COPD. Table 4 shows the inflammatory markers analysed. The group of patients with anaemia had significantly lower IL-6 values, but no differences were found in the other parameters. A direct correlation was observed between the haemoglobin levels and serum IL-6 levels (Kendall's Tau-b=0.16; P=.018, and Spearman's Rho=−0.20; P=.021). Correlations with the other inflammatory parameters analysed were not significant.
Patient Distribution According to Grade of Obstruction.
| Grade of obstruction | ||||
| Mild (n=4) | Moderate (n=54) | Severe (n=38) | Very severe (n=34) | |
| Patients without anaemia | 4 (100%) | 51 (94.4%) | 36 (94.7%) | 31 (91.2%) |
| Patients with anaemia | 0 | 3 (5.6%) | 2 (5.3%) | 3 (8.8%) |
The percentages in each cell correspond to the percentage of patients for each obstruction grade.
Blood High-Sensitivity CRP, IL-6, IL-8, TNF-α and Fibrinogen Values. Differences Between Groups.
| Patients without anaemia (n=122) | Patients with anaemia (n=8) | P | |
| High-sensitivity CRP, mg/L | 7.1±17.1 (n=117) | 16.8±26.9 (n=8) | .28 |
| IL-6, pg/mL | 23.7±91.4 (n=119) | 5.3±5 (n=8) | .003 |
| IL-8, pg/mL | 32.8±72.8 (n=120) | 28.8±21.7 (n=8) | .72 |
| TNF-α, pg/mL | 5.5±2 (n=121) | 6.1±2.1 (n=8) | .41 |
| Fibrinogen, mg/dL | 371.4±97.9 (n=117) | 424.5±132.5 (n=6) | .18 |
The values are shown as mean±SD.
In our study, the prevalence of anaemia was 6.2%, which is lower than most figures published to date.5–11 All show a prevalence above 10%, except for those by Watz et al.8 and Nowinski et al.12 These differences in prevalence are most likely due to methodological differences between studies: most are retrospective studies,6,7 analysis of different populations: stable or exacerbated,5,8,9 outpatients6–9 or inpatients10,11; different definitions of anaemia5,8; different grades of obstruction with FEV1 between 37%5 and 56%8; and confounding factors included in the analysis, such as heart failure, renal failure or associated neoplasms.
There are reports that show that the prevalence and severity of anaemia in the general population increase with age and are related with the patient's underlying condition. Thus, in the study by Guralnik et al.,20 the prevalence of anaemia of unknown cause in the group aged between 50 and 64 years was 4.4% in men and 6.8% in women, while in the group aged between 64 and 74 years it was 7.8% in men and 8.5% in women. These values are similar to those found in our study, where the mean age was 64.2 years (SD=8.4).
The main aim of our study was to determine the prevalence of anaemia not attributable to other causes in COPD patients, and for this reason, cases with other known causes of anaemia, as described in the study methods, were excluded. The Charlson index for the population included in our study is around 2. Of these, 43% of patients had hypertension, 33% hypercholesterolaemia and 16% diabetes mellitus. These data are very similar to those of other studies evaluating the presence of comorbidities in COPD patients.21,22 This confirms that our sample is a representative population of patients with COPD and their spectrum of the most common comorbidities, since only those that could cause anaemia were excluded.
The anaemia was generally mild (mean haemoglobin values of 11.9±0.95g/dL) and its characteristics were consistent with anaemia of chronic disease, similar to other previous studies.5,7,9 Having applied very strict exclusion criteria, our study suggests that the prevalence of anaemia not attributable to other causes in stable COPD patients is lower than found up to now in the literature, and may be close to the prevalence of anaemia in the general population in the same age range.
Although it did not reach statistical significance, patients with anaemia had a tendency to have more severe COPD in terms of FEV1, with a prevalence of anaemia in patients with very severe COPD close to 9%. Most previous studies do not describe a relationship between the prevalence of anaemia and the severity of the COPD, except for the one by Watz et al.,8 in which the prevalence of anaemia was higher in patients with very severe COPD (14%), and that of Boutou et al.,9 in which the group with anaemia had a lower FEV1 than the group without anaemia.
Few studies have analysed the impact of anaemia on patients with COPD. The efficiency of gas exchange depends on haemoglobin levels; if these fall, that exchange and, therefore, the exercise capacity could worsen.23 In our study, no differences were observed with respect to the dyspnoea scale, or in metres covered in the 6-minute walk test, unlike two previous studies that analysed these parameters, in which patients with anaemia had a higher grade of dyspnoea and covered less distance in the 6-minute walk test.
Although there is increasing data in relation to the role of systemic inflammation in COPD, few studies mention the physiopathology of COPD-associated anaemia. In theory, this could be similar to other chronic inflammatory diseases, where it has been postulated that its origin could be in an immunological and inflammatory response, mediated by TNF-α, IL-1, IL-6 and interferon gamma, which would generate changes in iron metabolism, reducing its intestinal absorption and altering haematopoiesis.2,24 In patients with COPD, there is an increase in these mediators, which could be considered as factors related with the onset of anaemia associated with this disease.25 In our study, the anaemia presented findings consistent with anaemia of chronic disease, and as in two other previous studies,5,25 it presented with low iron and significantly higher erythropoietin levels, which support the theory of peripheral resistance to the action of erythropoietin mediated by an inflammatory component. These findings, which are common to other chronic diseases that present with anaemia, are important, as studies have recently been carried out suggesting that the correction of iron deficiency in patients with heart failure could improve their prognosis, thereby opening new therapeutic targets to be studied in the future.26
Only one previous paper5 compared interleukin and CRP values in COPD patients with and without anaemia in relation to healthy controls. In this study, IL-6 and CRP were significantly higher in COPD patients than in controls, with no differences found in the other interleukins (IL-8 and IL-10). In the COPD group, patients with anaemia had significantly higher CRP values than non-anaemic patients, but no differences were found in the IL-6, IL-8 and IL-10 values. In our study, we found a non-significant increase in CRP levels, and patients with anaemia had significantly lower IL-6 values and a direct correlation between the haemoglobin and IL-6 levels. We did not find a physiopathological explanation for these findings, which should be confirmed in future studies.
Moreover, there are other added factors that influence the erythropoietic response and which also may be present in COPD patients.27 Thus, it is known that certain drugs may influence haemoglobin levels, such as angiotensin-converting-enzyme inhibitors and angiotensin II receptor inhibitors, which cause a decrease in haemoglobin and erythropoietin levels. These drugs are often used for the treatment of hypertension and, according to some studies, the prevalence of this condition in COPD patients could be around 50%.28 Other treatments, such as theophylline, may also influence erythropoiesis, by a complex mechanism of apoptosis induced by the drug itself.29 In our study, 50% of patients with anaemia were on treatment with angiotensin II receptor blockers compared to 11.5% of the group without anaemia (P=.03), although no differences were found in relation to the use of other drugs such as angiotensin-converting-enzyme inhibitors and theophyllines.
Our study is not free of limitations, especially those that could be derived from the sample size or the use of some exclusion criteria not included in previous studies, which could limit the comparison of our data with previously published results. Despite this, our data are interesting, as they show a lower prevalence of COPD-associated anaemia than previously described and open the door to physiopathological theories that could explain the onset of anaemia in these patients. Further studies with larger COPD populations and stricter inclusion criteria are required in order to eliminate confounding factors, and which allow a better understanding of the onset of anaemia in these patients from a physiopathological point of view and of the clinical and prognostic implications.
FundingThis study was partially funded by GlaxoSmithKline S.A. (CRT114268).
Conflict of InterestsThe authors declare that they have no conflict of interests.
Please cite this article as: Comeche Casanova L, et al. Prevalencia de anemia asociada a la enfermedad pulmonar obstructiva crónica. Estudio de las variables asociadas. Arch Bronconeumol. 2013;49:383-387.