In 2008, the Spanish Society of Pulmonology (SEPAR) published the first guidelines in the world on the diagnosis and treatment of bronchiectasis. Almost 10 years later, considerable scientific advances have been made in both the treatment and the evaluation and diagnosis of this disease, and the original guidelines have been updated to include the latest therapies available for bronchiectasis. These new recommendations have been drafted following a strict methodological process designed to ensure quality of content, and are linked to a large amount of online information that includes a wealth of references. The guidelines are focused on the treatment of bronchiectasis from both a multidisciplinary perspective, including specialty areas and the different healthcare levels involved, and a multidimensional perspective, including a comprehensive overview of the specific aspects of the disease. A series of recommendations have been drawn up, based on an in-depth review of the evidence for treatment of the underlying etiology, the bronchial infection in its different forms of presentation using existing therapies, bronchial inflammation, and airflow obstruction. Nutritional aspects, management of secretions, muscle training, management of complications and comorbidities, infection prophylaxis, patient education, home care, surgery, exacerbations, and patient follow-up are addressed.
En 2008, la Sociedad Española de Neumología y Cirugía Torácica (SEPAR) publicó las primeras normativas del mundo sobre el diagnóstico y tratamiento de las bronquiectasias. Tras casi una década, muchos han sido los avances científicos en esta enfermedad, tanto en sus aspectos terapéuticos como en su valoración y diagnóstico. Por ello estas nuevas normativas sobre el tratamiento de las bronquiectasias en el adulto tratan de ofrecer al lector una actualización del conocimiento científico sobre las posibilidades terapéuticas en bronquiectasias, basándose en un estricto procedimiento metodológico que asegura la calidad del contenido de la misma, y en una amplia cantidad de información online que incluye abundante bibliografía. En estas normativas se ha enfocado el tratamiento de las bronquiectasias desde un punto de vista tanto multidisciplinar, que implica las especialidades y escalones asistenciales involucrados, como multidimensional que incluye todos y cada uno de los aspectos que definen a la enfermedad. Así, se establecen recomendaciones basadas en una exhaustiva revisión de la evidencia sobre los tratamientos de la etiología, de la infección bronquial en sus diferentes formas de presentación y con las diferentes terapias existentes, de la inflamación bronquial y de la obstrucción al flujo aéreo. Se revisan los aspectos nutricionales, el manejo de las secreciones, el entrenamiento muscular, el manejo de las complicaciones y comorbilidades, la profilaxis de la infección, los aspectos educacionales, el manejo del paciente en el domicilio, el tratamiento quirúrgico, las agudizaciones y el seguimiento de los pacientes.
Non-cystic fibrosis (CF) bronchiectasis (hereinafter, bronchiectasis [BE]) is the third most common chronic inflammatory disease of the airways after asthma and chronic obstructive pulmonary disease (COPD), and is closely related to both. In 2008, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) became the first scientific society to establish guidelines on the diagnosis and treatment of BE, including CF.1 More than 8 years later, the scientific evidence on BE has become clearer on a number of major issues, and the findings of recent studies have compelled us to publish these new guidelines, which, in order to provide the reader with more specific information, will focus solely on BE in adults. This section concerns the treatment of BE. The guidelines have been prepared with the advice of an expert in methodology. A Delphi system was used to create the list of topics, prioritizing the clinical questions (Annex 1); key clinical questions were structured according to the PICO (Patient-Intervention-Comparison-Outcome) system, and appear as an annex at the end of the manuscript (Annex 3). Finally, the certainty of the evidence and the strength of the recommendations were established following the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system (Annexes 1 and 2).
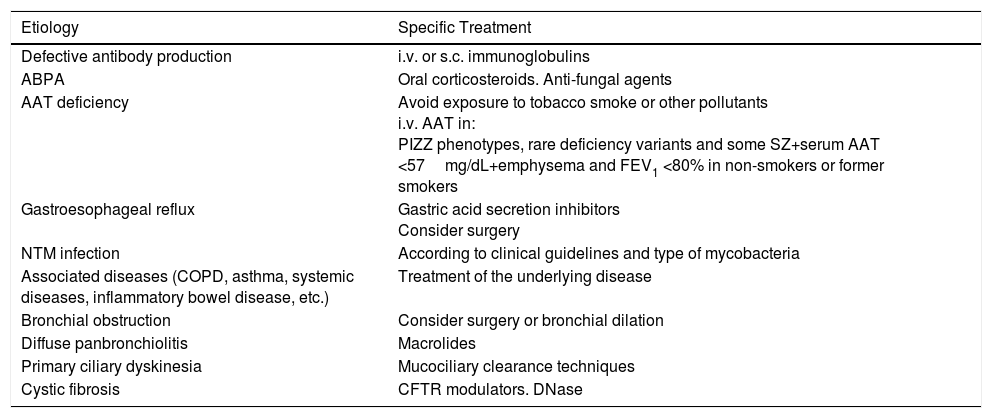
TreatmentTreatment of the EtiologyIt is important to identify BE etiologies that have specific treatment, in order to start therapy as soon as possible to control symptoms and prevent progression of lung damage (Table 1). Treatment of the underlying disease should be reviewed at each clinical assessment.1
Causes of Bronchiectasis With Specific Treatment.
| Etiology | Specific Treatment |
|---|---|
| Defective antibody production | i.v. or s.c. immunoglobulins |
| ABPA | Oral corticosteroids. Anti-fungal agents |
| AAT deficiency | Avoid exposure to tobacco smoke or other pollutants i.v. AAT in: PIZZ phenotypes, rare deficiency variants and some SZ+serum AAT <57mg/dL+emphysema and FEV1 <80% in non-smokers or former smokers |
| Gastroesophageal reflux | Gastric acid secretion inhibitors Consider surgery |
| NTM infection | According to clinical guidelines and type of mycobacteria |
| Associated diseases (COPD, asthma, systemic diseases, inflammatory bowel disease, etc.) | Treatment of the underlying disease |
| Bronchial obstruction | Consider surgery or bronchial dilation |
| Diffuse panbronchiolitis | Macrolides |
| Primary ciliary dyskinesia | Mucociliary clearance techniques |
| Cystic fibrosis | CFTR modulators. DNase |
AAT: α-1 antitrypsin; ABPA: allergic bronchopulmonary aspergillosis; CFTR: cystic fibrosis transmembrane conductance regulator; COPD: chronic obstructive pulmonary disease; i.v.: intravenous; NTM: non-tuberculous mycobacteria; s.c.: subcutaneous.
An association has been shown between chronic bronchial Pseudomonas aeruginosa (P. aeruginosa) infection and poorer prognosis in patients with BE.2–5 Based mainly on the benefit of P. aeruginosa eradication in CF, eradication in BE patients should also be attempted (Strong recommendation. Low quality evidence).6,7
Considerations:
- -
No P. aeruginosa eradication protocol (Table 2) has demonstrated superiority over another.
Table 2.Antimicrobial Treatment in Bronchial Infection in Patients with Bronchiectasis.
Clinical Situation Comments Treatment of the primary infection No eradication protocol has shown superiority over another
If 3 treatment strategies fail, the chronic infection protocol should be appliedFirst culture positive for P. aeruginosa (initial infection)
Ciprofloxacin 750mg/12h p.o. for 3 weeks
-→In the case of severe BE (E-FACED 6–9 points) and immunosuppressed patients, an inhaled antibiotic (colistimethate, tobramycin or azteronam) should be added from onset for 3 months
-→In the case of allergy or intolerance to ciprofloxacin, only inhaled antibiotic should be used for 3 months, except in the case of severe BE and immunosuppressed patients who, from the outset, should use 1 or 2 i.v. antibiotics with anti-pseudomonas activity for 2–3 weeks according to antibiotic susceptibility testing, in addition to inhaled antibiotic
-→If initial infection is detected in the exacerbation period, therapy should be initiated with i.v. antibiotics with anti-pseudomonas activity (see section on exacerbations)
Inhaled antibiotics that should be used in initial infections: aztreonam lysine (solution for inhalation) or colistimethate sodium solution for inhalation or tobramycin solution for inhalation (posology: see table of inhaled antibiotics)Perform sputum culture monthly for the first 3 months after completing ciprofloxacin treatment and every 2 months thereafter for 1 year
If P. aeruginosa presents again at any time during the first year in the sputum culture, consider eradication failure. In this case: (a) Add an inhaled antibiotic to the ciprofloxacin or (b) Repeat the same ciprofloxacin regimen+inhaled antibiotic, or (c) Switch to another combination (ciprofloxacin+an inhaled antibiotic not used in the first cycle)
If at least 2 strategies that include inhaled and oral antibiotics fail, simultaneous use of inhaled plus i.v. antibiotics is recommendedFirst culture positive for MRSA (initial infection)
Fusidic acid 500mg/12h p.o., cotrimoxazole 160/800mg/12h p.o., rifampicin 600mg/24h p.o., clindamycin 300mg/6h p.o., linezolid 600mg/12h p.o., tedizolid 200mg/24h p.o. or vancomycin (i.v. formulation administered via inhalation, continuous treatment, 250mg, twice daily)Different combinations of these oral antibiotics should be used simultaneously inhaled vancomycin may be added to any of these regimens
Duration: 2 weeks (tedizolid 6 days)
If eradication is not achieved, i.v. antibiotics against MRSA should be prescribed (tigecycline, teicoplanin, vancomycin±rifampicin), added or not to inhaled vancomycin
Do not use rifampicin and linezolid together due to decreased levels of the latterFirst culture positive for any PPM (initial infection)
Antibiotics p.o. according to antibiotic susceptibility testingDuration: 2 weeks
If eradication is not achieved, give another cycle with the same antibiotic or switch to anotherTreatment of chronic infection Treatment protocols for chronic infection are varied and none has demonstrated superiority over another
Duration: maintain inhaled regimen as long as the benefit/risk is favorable (usually years)Chronic P. aeruginosa infection
In alphabetical order: aztreonam lysine (solution for inhalation) or bciprofloxacin (dry powder or solution for inhalation) or acolistimethate (dry powder or solution for inhalation) or cgentamicin (i.v. formulation administered via inhalation) or tobramycin (dry powder or solution for inhalation)In moderate-severe lung impairment or insufficient response in on/off cycles
-→ Use inhaled antibiotics continuously, alternating/rotating them with no breaks between them or with off intervals shorter than 28 days
-→If poor clinical control persists despite inhaled treatment, add an oral or i.v. antibiotic with anti-pseudomonas activity according to sensitivity, on demand or in cyclesChronic MRSA infection
dVancomycin (i.v. formulation administered via inhalation), continuous treatment, 250mg, twice daily
Chronic Stenotrophomonas maltophilia infection
Colistimethate, solution for inhalationIn the case of insufficient response or intolerance, add cotrimoxazole 160/800mg/12h p.o. to the vancomycin or switch this to cotrimoxazole
In the case of insufficient response or intolerance, add cotrimoxazole 160/800mg/12h p.o. to the colistimethate or switch this to cotrimoxazoleChronic infection with other PPM
cGentamicin is an i.v. formulation used via the inhaled route (80mg, twice daily, continuous treatment)
or
Any of the inhaled antibiotics used in chronic P. aeruginosa infectionIf the response is insufficient: (a) Try efficacy with other i.v. formulations of antibiotics administered via inhalation or (b) Add (or switch) the inhaled antibiotic for an oral one according to the antibiotic sensitivities of the PPM causing the infection BE: bronchiectasis; i.v.: intravenous; MRSA: methicillin-resistant Staphylococcus aureus; p.o.: orally; PPM: potentially pathogenic microorganisms.
The antibiotics used via the inhaled route are listed in alphabetical order, not order of priority.
aThe colistimethate sodium dose depends on the efficacy of the nebulizer used. Lower doses (1mU, twice daily) should be used with an adaptive aerosol delivery nebulizer, such as the I-neb, although there is a lack of lung deposition studies to confirm this.
- -
For other potentially pathogenic microorganisms (PPM), the decision to apply an eradication treatment in initial infection should be made on an individual basis according to the patient's symptoms and the PPM in question, since there is no strong evidence on its contribution to the pathogenesis of BE (Table 2).
The main aim of antibiotic treatment of chronic bronchial infection is to maximize reduction of bacterial density to break the vicious pathogenic circle of airway infection-inflammation, reducing both as far as possible and thereby slowing clinical–functional decline. Prolonged antibiotic treatment is recommended in the following situations: (a) In all patients who present with chronic bronchial P. aeruginosa infection (Strong recommendation. Moderate quality evidence); (b) In patients with chronic bronchial infection due to other PPMs, who also present at least 2 exacerbations or 1 hospitalization for exacerbation during the previous year, marked decline in lung function or deterioration in quality of life evidenced by an increase in sputum volume or purulence, dyspnea or cough (Strong recommendation. Low quality evidence)8 (Table 2).
Inhaled rather than systemic antibiotics are recommended due to their high effectiveness (significant reduction of bacterial load, decrease in local inflammation, improved quality of life and reduction in the number of exacerbations) and good safety profile (high antibiotic concentrations at the infection site with minimal systemic side effects and lower rate of resistance) (Strong recommendation. Moderate quality evidence).9
Considerations:
- -
Treatment protocols for chronic bronchial infection are very varied and none has demonstrated superiority over another.
- -
If poor control persists, or there is inadequate clinical progress despite inhaled treatment, on demand or cyclical (every 1–2 months) oral or intravenous antibiotic therapy should be added.
- -
Since inhaled antibiotics achieve high concentrations the airways, they should be chosen on the basis of the type of PPM causing the infection, but not on their antibiotic sensitivity.
- -
This treatment should be prescribed indefinitely as long as the risk/benefit balance is favorable, since many patients can worsen clinically when it is discontinued. The effectiveness of treatment in controlling the infection should be monitored by achieving and maintaining the least purulent sputum possible and reducing exacerbations. Cultures with bacterial counts can also help to assess treatment efficacy.
- -
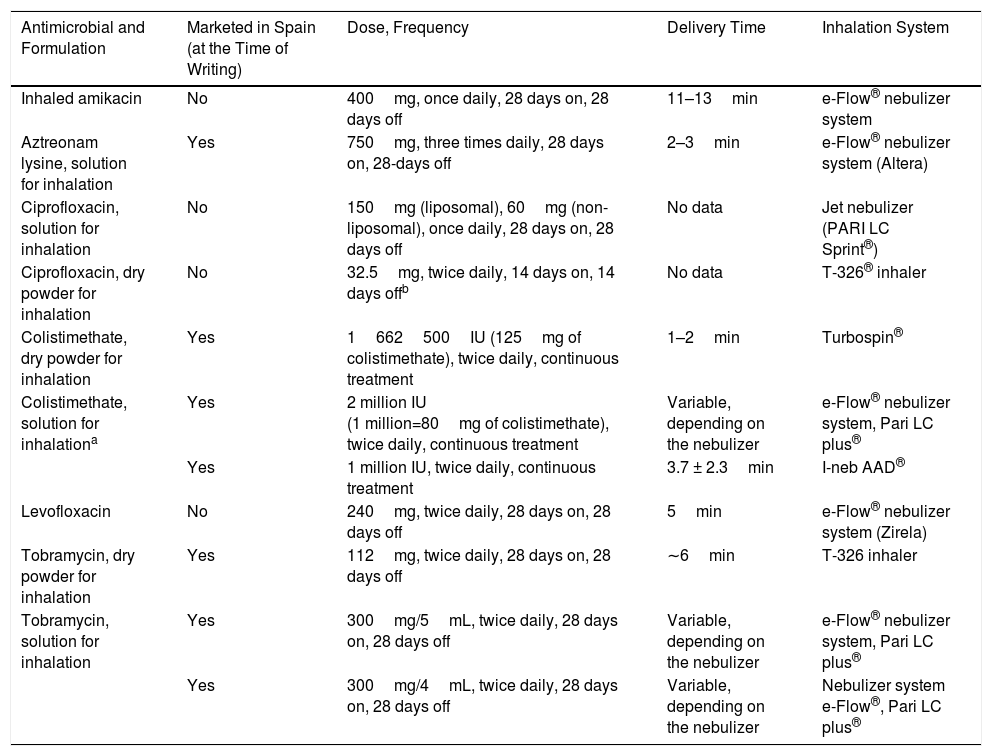
Inhaled antibiotics currently marketed in Spain are: colistimethate sodium (available for nebulization and in dry powder), tobramycin (solution for nebulizer and as powder for inhalation) and aztreonam lysine for inhalation. The official indication for all these is currently chronic bronchial P. aeruginosa infection in patients with CF (but not BE), although their use in BE is very widespread. In two clinical trials conducted with aztreonam lysine10 and colistimethate sodium,11 respectively, a beneficial antibacterial effect was found that did not translate into a clinical benefit, except in the subgroup of patients treated with colistimethate sodium with good compliance (more than 80% of the doses). Aztreonam lysine presented more adverse effects than placebo. In a clinical trial, ciprofloxacin dry powder, in 14-day cycles, demonstrated clinical efficacy with a good safety profile (RESPIRE-1),12 although these results were not corroborated by another study with a similar design (RESPIRE-2).13 Likewise, another clinical trial (ORBIT-4) showed that ciprofloxacin in solution for inhalation, in 28-day cycles, showed clinical efficacy and a good safety profile, but again, a similarly-designed study was unable to confirm these clinical efficacy outcomes (ORBIT-3).14 New formulations of inhaled antibiotics (liposomal amikacin, levofloxacin in solution and ciprofloxacin solution for inhalation and dry powder) could be marketed in Spain in the near future (Table 3).
Table 3.Inhaled Antimicrobials.
Antimicrobial and Formulation Marketed in Spain (at the Time of Writing) Dose, Frequency Delivery Time Inhalation System Inhaled amikacin No 400mg, once daily, 28 days on, 28 days off 11–13min e-Flow® nebulizer system Aztreonam lysine, solution for inhalation Yes 750mg, three times daily, 28 days on, 28-days off 2–3min e-Flow® nebulizer system (Altera) Ciprofloxacin, solution for inhalation No 150mg (liposomal), 60mg (non-liposomal), once daily, 28 days on, 28 days off No data Jet nebulizer (PARI LC Sprint®) Ciprofloxacin, dry powder for inhalation No 32.5mg, twice daily, 14 days on, 14 days offb No data T-326® inhaler Colistimethate, dry powder for inhalation Yes 1662500IU (125mg of colistimethate), twice daily, continuous treatment 1–2min Turbospin® Colistimethate, solution for inhalationa Yes 2 million IU
(1 million=80mg of colistimethate), twice daily, continuous treatmentVariable, depending on the nebulizer e-Flow® nebulizer system, Pari LC plus® Yes 1 million IU, twice daily, continuous treatment 3.7 ± 2.3min I-neb AAD® Levofloxacin No 240mg, twice daily, 28 days on, 28 days off 5min e-Flow® nebulizer system (Zirela) Tobramycin, dry powder for inhalation Yes 112mg, twice daily, 28 days on, 28 days off ∼6min T-326 inhaler Tobramycin, solution for inhalation Yes 300mg/5mL, twice daily, 28 days on, 28 days off Variable, depending on the nebulizer e-Flow® nebulizer system, Pari LC plus® Yes 300mg/4mL, twice daily, 28 days on, 28 days off Variable, depending on the nebulizer Nebulizer system
e-Flow®, Pari LC plus® - -
Intravenous antibiotic formulations delivered via inhalation should not be used if the same antibiotic is available in a formulation for inhalation. There is no guarantee that the preparation is identical to that approved by the corresponding regulatory agencies; therefore, these formulations must be administered with caution, as they may be poorly tolerated and present a higher risk of adverse effects.
- -
Patients who use inhaled antibiotics on alternate months and present clinical worsening during the off-months (28-day periods) could benefit from using them continuously, alternating or rotating them with another antibiotic with no off-period, or shorter breaks (14 days). Colistimethate sodium is administered on a continuous basis (with no need for on/off cycles), on account of the low rate of resistance of P. aeruginosa to this antibiotic.
- -
Rotation or alternation of antibiotics could be useful to minimize the development of resistances. There is no evidence that inhalation of 2 concomitant antibiotics is more effective than using 1 alone, so this practice should only be used in particularly refractory patients (multiresistant microorganisms).
- -
The following therapeutic order is considered appropriate: bronchodilators, hypertonic saline solution (HSS), physiotherapy and inhaled antibiotics. Mesh nebulizers and dry powder inhalers are more effective than jet nebulizers. Ultrasonic nebulizers should not be used as they may inactivate the antibiotic. It is very important to clean and disinfect nebulizers properly after use. Dry powder and mesh nebulizers can administer the antibiotic faster than jet nebulizers, and this encourages treatment compliance. Similarly, the risk of infection from the device is minimized in dry powder inhalers.
- -
Since they can cause bronchospasm, dyspnea or cough, the first dose should be given under supervision following bronchodilation.
- -
Extreme caution is needed in patients with recurrent hemoptysis, major bronchial hyperresponsiveness (BHR) and severely impaired lung function. In patients with significant renal or hearing impairment, it is preferable to use antibiotics other than colistimethate and aminoglycosides, and to perform annual blood biochemistry tests, including a renal function panel.
In addition to their antibiotic action, macrolides can modulate bronchial inflammation and interfere in biofilm formation. They can reduce the number of exacerbations, as well as the amount of sputum, improve quality of life and attenuate lung function decline. They are recommended in patients with clinically stable BE but with at least 2 annual exacerbations despite appropriate background treatment (Strong recommendation. High quality evidence).15–17
Considerations:
- -
This situation usually appears in patients with chronic bronchial infection due to various PPMs, especially P. aeruginosa, and abundant expectoration or difficult clinical control despite antibiotic treatment.
- -
Of the different dosage regimens published, 500mg of azithromycin, 3 times weekly on non-consecutive days is generally the most convenient.
- -
The most common adverse effects are gastrointestinal, and are usually transient. Before initiating macrolide treatment, electrocardiogram (prolongation of the QT segment), laboratory tests (including liver function), and mycobacteria culture should be performed; if abnormalities appear, the antibiotics should not be administered due to the risk of inducing macrolide-resistant strains. Their use may increase resistance to common macrolide-sensitive PPMs.
- -
Their clinical efficacy should be re-evaluated every 6 months based on the decrease in the number of exacerbations.
Routine use is not recommended, except in patients with BHR, asthma or significant bronchorrhea that cannot be controlled with other treatments. Care should be taken with inhaled corticosteroid treatment in patients with chronic bronchial infection caused by PPM, as these drugs can increase susceptibility to infection (Strong recommendation. Low quality evidence).18
Other Anti-Inflammatory TreatmentsThere is scant evidence of the clinical efficacy of other anti-inflammatory treatments, such as neutrophil elastase inhibitors, anti-leukotrienes, phosphodiesterase-4 inhibitors or statins. Therefore, routine use of these is not recommended, except in the case of concomitant COPD, asthma or other comorbidities (Weak recommendation. Low quality evidence).
Treatment of Airflow ObstructionThe use of short-acting beta-agonists (salbutamol or terbutaline) is recommended before respiratory physiotherapy to facilitate airway clearance (AC) and the use of inhaled antibiotics or HSS (Strong recommendation. Moderate quality evidence).
The use of long-acting beta-agonists is recommended in patients who present with symptomatic airflow obstruction, provided the advantages outweigh the adverse effects, and to step down inhaled steroid doses (Strong recommendation. Moderate quality evidence).19
There is no information on the use of anti-cholinergics, so they should only be used in cases of concomitant asthma or COPD, or in particular cases in which other treatments have not produced the desired effect.
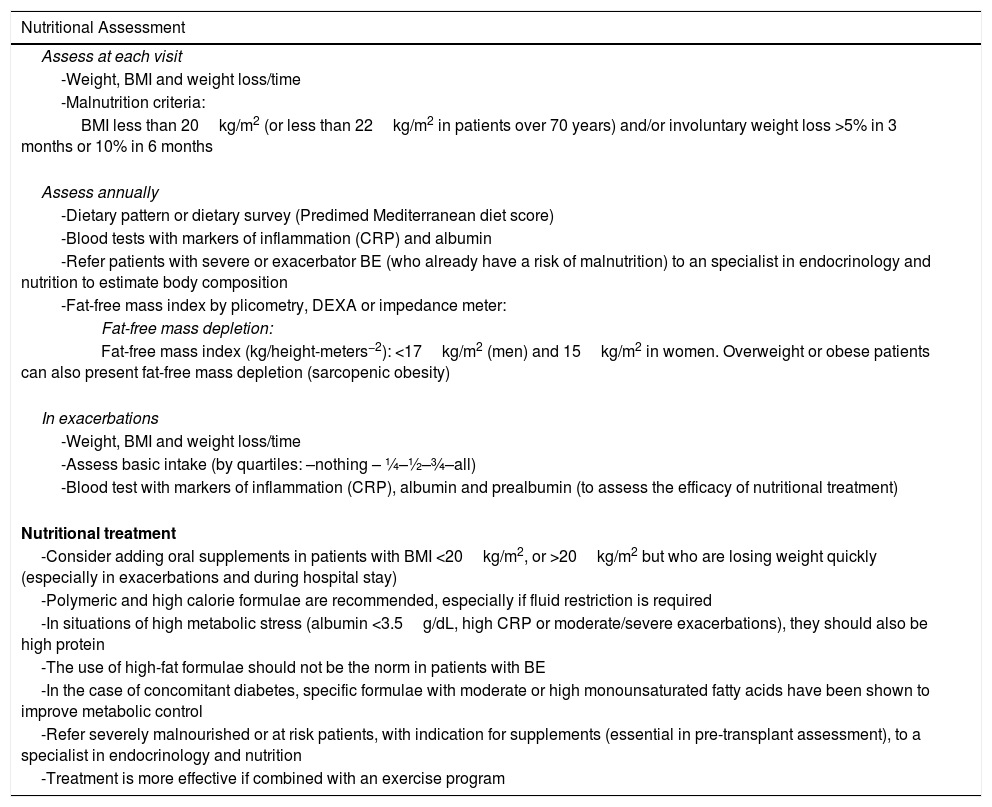
Nutritional AspectsPatients with BE are at risk of malnutrition (especially fat-free mass) because they have greater energy requirements (due to the increased work of breathing and chronic inflammation) and intake may be reduced due to anorexia (especially during exacerbations). The body mass index (BMI) is an independent marker of survival in BE (<20kg/m2) and fat-free mass depletion is associated with complications and higher mortality. Nutritional assessment should form part of the comprehensive evaluation of patients with BE; it is essential to measure the BMI at each visit, and to assess intake and weight loss over time. This is particularly important in patients with advanced disease and during exacerbations. It is also advisable to measure the body composition (Strong recommendation. Moderate quality evidence). Table 4 summarizes the nutritional assessment and treatment measures.20
Nutritional Assessment and Treatment.
| Nutritional Assessment |
|---|
| Assess at each visit |
| -Weight, BMI and weight loss/time |
| -Malnutrition criteria: |
| BMI less than 20kg/m2 (or less than 22kg/m2 in patients over 70 years) and/or involuntary weight loss >5% in 3 months or 10% in 6 months |
| Assess annually |
| -Dietary pattern or dietary survey (Predimed Mediterranean diet score) |
| -Blood tests with markers of inflammation (CRP) and albumin |
| -Refer patients with severe or exacerbator BE (who already have a risk of malnutrition) to an specialist in endocrinology and nutrition to estimate body composition |
| -Fat-free mass index by plicometry, DEXA or impedance meter: |
| Fat-free mass depletion: |
| Fat-free mass index (kg/height-meters−2): <17kg/m2 (men) and 15kg/m2 in women. Overweight or obese patients can also present fat-free mass depletion (sarcopenic obesity) |
| In exacerbations |
| -Weight, BMI and weight loss/time |
| -Assess basic intake (by quartiles: –nothing – ¼–½–¾–all) |
| -Blood test with markers of inflammation (CRP), albumin and prealbumin (to assess the efficacy of nutritional treatment) |
| Nutritional treatment |
| -Consider adding oral supplements in patients with BMI <20kg/m2, or >20kg/m2 but who are losing weight quickly (especially in exacerbations and during hospital stay) |
| -Polymeric and high calorie formulae are recommended, especially if fluid restriction is required |
| -In situations of high metabolic stress (albumin <3.5g/dL, high CRP or moderate/severe exacerbations), they should also be high protein |
| -The use of high-fat formulae should not be the norm in patients with BE |
| -In the case of concomitant diabetes, specific formulae with moderate or high monounsaturated fatty acids have been shown to improve metabolic control |
| -Refer severely malnourished or at risk patients, with indication for supplements (essential in pre-transplant assessment), to a specialist in endocrinology and nutrition |
| -Treatment is more effective if combined with an exercise program |
BE: bronchiectasis; BMI: body mass index; CRP: C-reactive protein; DEXA: dual-energy X ray absorptiometry.
AC techniques are safe and recommended in adult patients with clinically stable BE with productive cough, because they significantly improve quality of life, especially hypersecretory patients or those with frequent exacerbations (Strong recommendation. Low quality evidence).21
Considerations:
- -
The choice of technique should be based on the patient's preference, their ability, comorbidity and interference in daily life.
- -
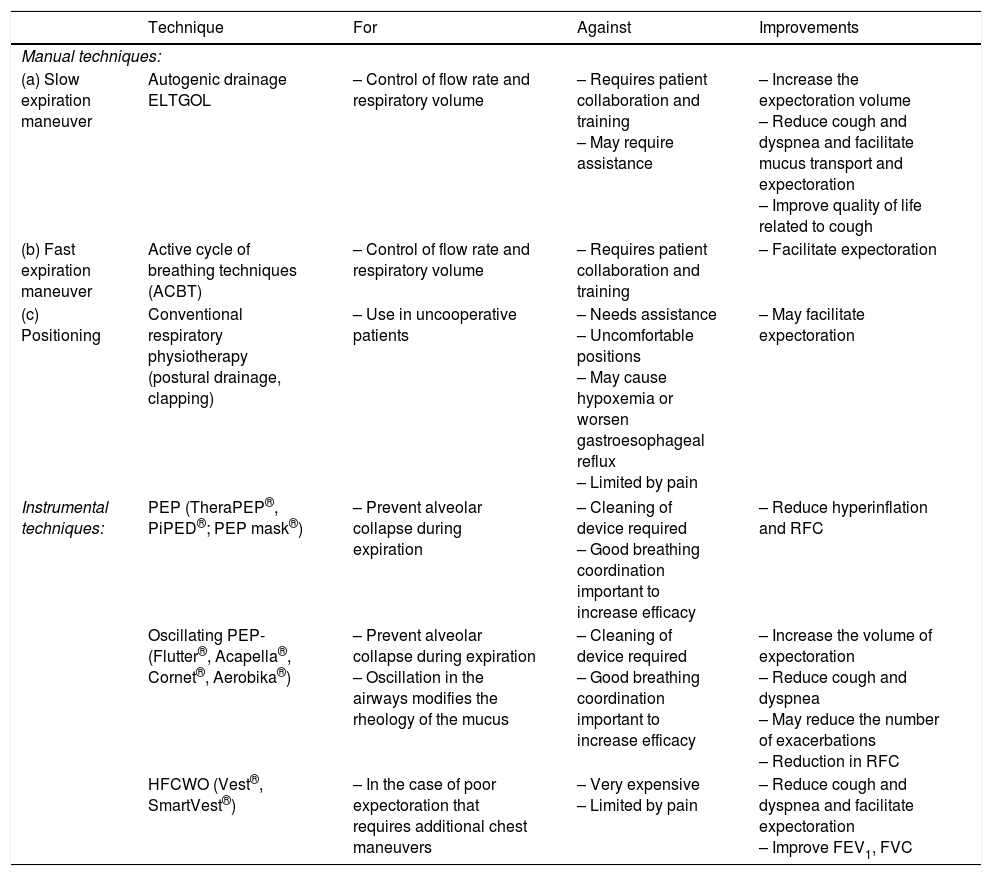
AC should form part of an overall training program, and should be carried out at least once daily or as required. AC techniques can be either manual (autogenic drainage, slow expiration with glottis opened and active cycle of breathing techniques [ACBT]) or instrumental (positive expiratory pressure [PEP], oscillating positive expiratory pressure [OPEP] and high frequency chest wall oscillation [HFCWO]). All reduce the symptoms of dyspnea and cough and facilitate expectoration. The use of OPEP also increases the volume of expectoration and may reduce the number of exacerbations. HFCWO performed for 15 days may improve dynamic lung function values (forced expiratory volume in the first second [FEV1] and forced vital capacity). Slow expiration with glottis opened, PEP and OPEP may reduce pulmonary hyperinflation, decreasing the functional residual capacity. Finally, a significant reduction in peripheral airway resistance has been observed (Table 5).
Table 5.Standard Physiotherapy Techniques in Bronchiectasis.
Technique For Against Improvements Manual techniques: (a) Slow expiration maneuver Autogenic drainage
ELTGOL– Control of flow rate and respiratory volume – Requires patient collaboration and training
– May require assistance– Increase the expectoration volume
– Reduce cough and dyspnea and facilitate mucus transport and expectoration
– Improve quality of life related to cough(b) Fast expiration maneuver Active cycle of breathing techniques (ACBT) – Control of flow rate and respiratory volume – Requires patient collaboration and training – Facilitate expectoration (c) Positioning Conventional respiratory physiotherapy (postural drainage, clapping) – Use in uncooperative patients – Needs assistance
– Uncomfortable positions
– May cause hypoxemia or worsen gastroesophageal reflux
– Limited by pain– May facilitate expectoration Instrumental techniques: PEP (TheraPEP®, PiPED®; PEP mask®) – Prevent alveolar collapse during expiration – Cleaning of device required
– Good breathing coordination important to increase efficacy– Reduce hyperinflation and RFC Oscillating PEP-(Flutter®, Acapella®, Cornet®, Aerobika®) – Prevent alveolar collapse during expiration
– Oscillation in the airways modifies the rheology of the mucus– Cleaning of device required
– Good breathing coordination important to increase efficacy– Increase the volume of expectoration
– Reduce cough and dyspnea
– May reduce the number of exacerbations
– Reduction in RFCHFCWO (Vest®, SmartVest®) – In the case of poor expectoration that requires additional chest maneuvers – Very expensive
– Limited by pain– Reduce cough and dyspnea and facilitate expectoration
– Improve FEV1, FVCFVC: forced vital capacity; ELTGOL: slow expiration with the glottis open in a lateral posture; FEV1: forced expiratory volume in the first second; RFC: residual functional capacity; HFCWO: high frequency chest wall oscillation; OPEP: oscillating positive expiratory pressure techniques; PEP: positive expiratory pressure techniques.
- -
AC is contraindicated in unstable disease, hemoptysis, bronchospasm, intracranial hypertension, pneumothorax and recent eye surgery. Techniques applied directly on the chest are contraindicated in bone abnormalities, such as metastasis, osteoporosis or rib fractures.
Physical training, within pulmonary rehabilitation programs, is recommended in stable patients with dyspnea greater than 1 on the mMRC scale (Strong recommendation. Moderate quality evidence).22
Considerations:
- -
Thrice-weekly programs (for example, 2 hospital and 1 home session), supervised, for at least 8 weeks, have been shown to be effective.
- -
The aerobic exercise generally consists of working at moderate or high intensities (75% maximum speed of the incremental shuttle walk test [ISWT], or at least 60%–70% of the maximum heart rate in the 6-min walk test, or 85% of the maximum oxygen consumption [V02max] for 30–45min using a cycle ergometer, treadmill or elliptic machine). Upper and lower limb strengthening exercises can be included with progressive load (starting at 60% of the maximum load, as well as strengthening the inspiratory muscles twice daily for 15min, starting the work at 30% of the maximum inspiratory pressure and increasing gradually by 5% each week to reach 60%). These interventions improve exercise tolerance (measured by ISWT or 6-MWT) and quality of life. The effects are maintained 12 weeks after the end of the exercise but are gradually reduced over the course of a year. The inspiratory muscle work could be related with a longer duration of effect. Pulmonary rehabilitation significantly reduces the number, but not the duration, of exacerbations or the length of antibiotic therapy needed to treat them.
There is insufficient evidence to recommend the routine use of mucolytics in BE (Strong recommendation. Low quality evidence).23
Considerations:
- -
High-dose bromhexine (30mg/8h) for 15 days together with an antibiotic could improve AC in patients during an exacerbation. In stable patients, it may be used in individuals over age 55 with daily expectoration greater than 30mL.
- -
Erdosteine (225mg/12h) for 15 days together with mucociliary clearance techniques has been shown to improve sputum purulence and lung function. This mucolytic is not currently marketed in Spain.
- -
In patients who present COPD in addition to BE, other mucolytics can be considered in combination with the usual treatment, such as acetylcysteine (dose 400–1800mg/day), carbocysteine (dose 1500–2700mg/day) or ambroxol (75mg/day) in the longer term (>10 months), as they can reduce exacerbations. DNase in aerosol is not useful (and may even be harmful) in patients with BE.
Hypertonic substances are recommended in patients with BE with expectoration greater than 10mL per day or ≥2 exacerbations per year despite correct background treatment (Strong recommendation. Moderate quality evidence).24
Considerations:
- -
Use inhalation of 5mL of HSS at a concentration of 6% or 7%, once or twice daily for at least 3 months to note its effects. Inhalation of HSS has been found to facilitate bronchial drainage, decrease viscosity, improve FEV1 and quality of life, and reduce exacerbations and antibiotic use.
- -
Prolonged use of inhaled dry powder mannitol at a dose of 400mg/12h (10×40mg capsules) was shown to improve quality of life and increase time to the first exacerbation in patients with BE, but is not yet marketed in Spain. Before using a hypertonic substance, a substance tolerance test should be performed by forced spirometry before and after inhalation. Administration should be discontinued in the event of FEV1 decreases ≥15%.
- -
Likewise, in patients who cannot tolerate HSS, the formulation with added hyaluronic acid is an alternative to consider or, failing that, a more isotonic saline solution (diluting it with 0.9% SS or trying lower HSS concentrations, such as 3%) A greater benefit is obtained with hypertonic substances preceded by a bronchodilator and followed by respiratory physiotherapy.
Oxygen therapy should be prescribed following the usual indications for chronic airway diseases. In clinically stable phases, non-invasive mechanical ventilation is indicated in chronic respiratory failure as a coadjuvant to other pulmonary rehabilitation and physiotherapy techniques and as a bridge to lung transplantation. The most suitable non-invasive mechanical ventilation modality is bi-level positive airway pressure ventilation (Strong recommendation. Low quality evidence).
Treatment of ComplicationsComplications (life-threatening hemoptysis, pneumothorax, amyloidosis, sepsis, renal failure, etc.) should be treated following the current guidelines in each case. In hemoptysis, the use of inhaled drugs and respiratory physiotherapy should be avoided until 48h after resolution. The appearance of hemoptoic sputum usually indicates the presence of an infection that should be treated with antibiotic therapy.
SurgerySurgery (lobectomies or segmentectomies) is the only curative treatment in localized BEs that are refractory to clinical management, provided that underlying diseases that contribute to onset have been ruled out. Surgery with palliative intent is also indicated in cases of severe hemoptysis with ineffective embolization, or of abscessed areas that cannot be cured with antibiotic treatment. Factors such as the existence of residual BE, P. aeruginosa or non-tuberculous mycobacteria infection and immunosuppression can result in a poorer clinical response following surgery (Strong recommendation. Moderate quality evidence).25
Lung TransplantationIndicated in patients with advanced chronic lung disease or evidence of disease progression, with estimated survival less than 2 years once all available therapeutic resources have been exhausted, provided there is no absolute contraindication. There are no specific indications for lung transplant referral in patients with BE, although those of CF or the underlying disease are assumed if they have their own indications. Table 6 shows the main indications for transplantation.26
Indications for Lung Transplantation.
| FEV1 less than 30% or rapid loss of lung function in patients with severe impairment |
| Chronic respiratory failure or hypercapnia |
| Pulmonary hypertension (pulmonary artery systolic pressure >35mmHg) |
| Frequent severe exacerbations or complications such as pneumothorax or recurrent hemoptysis |
FEV1: forced expiratory volume in the first second.
A high proportion of patients with BE present comorbidities that must be identified and treated. Those with the greatest impact on BE patients are rhinosinusitis and/or nasal polyposis, hiatus hernia, anxiety and depression. Immunosuppressant and/or biological treatments can lead to a higher number of respiratory infections that require close monitoring.
Prophylaxis of InfectionAs in other chronic airway diseases, annual flu vaccination and 13-valent conjugated anti-pneumococcal vaccination are recommended. If the patient has already been vaccinated with the 23-valent anti-pneumococcal polysaccharide vaccine, it is recommended to wait a year until administration of the 13-valent conjugated anti-pneumococcal vaccine (Strong recommendation. Moderate quality evidence).27
Patient EducationEducation and supervision are important in understanding the disease, recognition of exacerbations and their initial self-management, administration of inhaled and intravenous antibiotics at home, maintenance and cleaning of equipment, administration of other treatments (oxygen, mechanical ventilation, etc.) and treatment compliance (Strong recommendation. Moderate quality evidence).
Home Intravenous Antibiotic TreatmentThe literature supports the efficacy of home intravenous antibiotic treatment for a wide variety of infections, provided that patients are carefully selected and trained. It has considerable advantages: reduced hospital admissions (thus avoiding nosocomial complications), reduced costs, no interference in the patient's daily routine, and improved quality of life. The patient's medical condition, infectious process and home should be carefully evaluated, and patient consent must be obtained. There are generally few complications, mostly related with the venous access. It is a safe and cost-effective treatment (Strong recommendation. Moderate quality evidence). Recommendations of guidelines published for home intravenous antibiotic treatment should be followed (Table 7). The home care team must consist of nursing staff coordinated by a pulmonologist and a cooperative group made up of pharmacists, physiotherapists and the primary care contact person. Medical care should be complemented by treatment in the hospital emergency department and health center.28
Selection Criteria for Home Intravenous Antibiotic Treatment.
| Inclusion |
| Clinically stable patient |
| Family or caregiver support |
| Highly motivated to have treatment at home |
| Patient and caregivers physically and mentally capacitated for home therapy |
| Home within the catchment area of the unit responsible for treatment |
| Home with adequate means: telephone, running water, refrigerator, etc. |
| Signed informed consent form |
| Exclusion |
| Possibility of effective oral treatment |
| Need for other inpatient treatment |
| Non-compliant patients, with psychosocial problems or drug addiction |
| Instability or severity criteria |
Exacerbation is defined as an acute sustained clinical deterioration characterized by an increase in the usual cough and changes in the sputum characteristics consisting of increased purulence, volume or viscosity, which may be accompanied by an increase in dyspnea, fever, asthenia, poor general condition, anorexia, pleuritic chest pain, hemoptysis, changes in the respiratory examination, changes in the patient's usual treatment or a significant decline in lung function.
The frequency and severity of the exacerbations have a significant impact, not only on the patient's quality of life, but also on disease progression (clinical and functional decline, increase in morbidity and mortality and associated costs), especially in the more severe functional classes. The pathogens most often isolated during an exacerbation are P. aeruginosa, Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Moraxella catarrhalis and enterobacteria, although viruses may also be involved, as they have been reported in up to 25% of exacerbations. In the case of pneumonia, S. pneumoniae continues to be the most common microbiological cause.
Severity Criteria and AdmissionExacerbations are classified as mild or moderate when they can be controlled with oral antibiotic treatment, and are considered severe when they require intravenous antibiotic treatment or hospitalization.29 Those that present with at least one of the following conditions are also considered severe: exacerbated acute or chronic respiratory failure, significant deterioration in oxygen saturation, high temperature or other criteria for sepsis, frank hemoptysis or significant deterioration in lung function. Exacerbations that present with hemodynamic instability, altered level of consciousness or need for admission to an intensive or intermediate care unit are considered very severe.
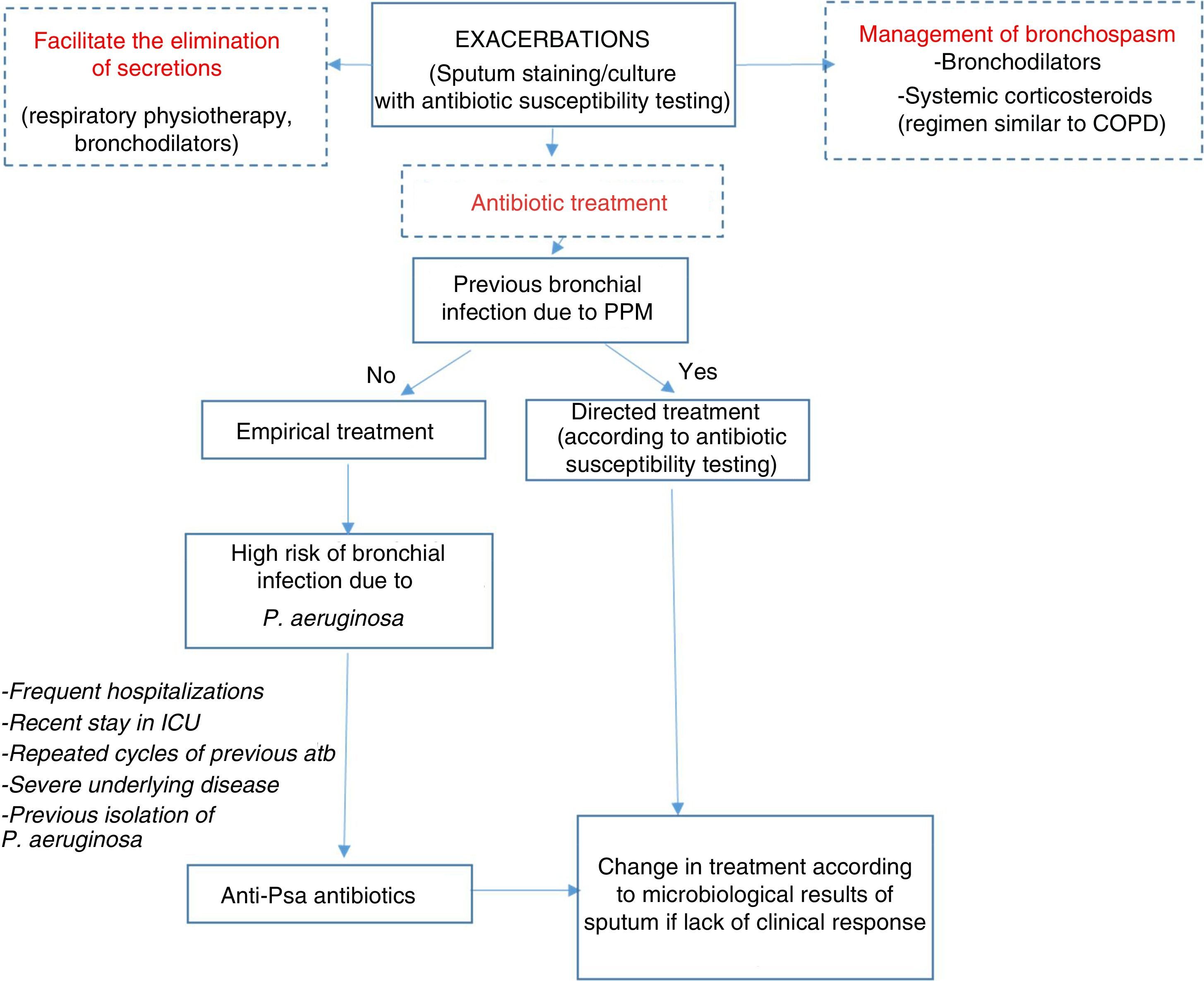
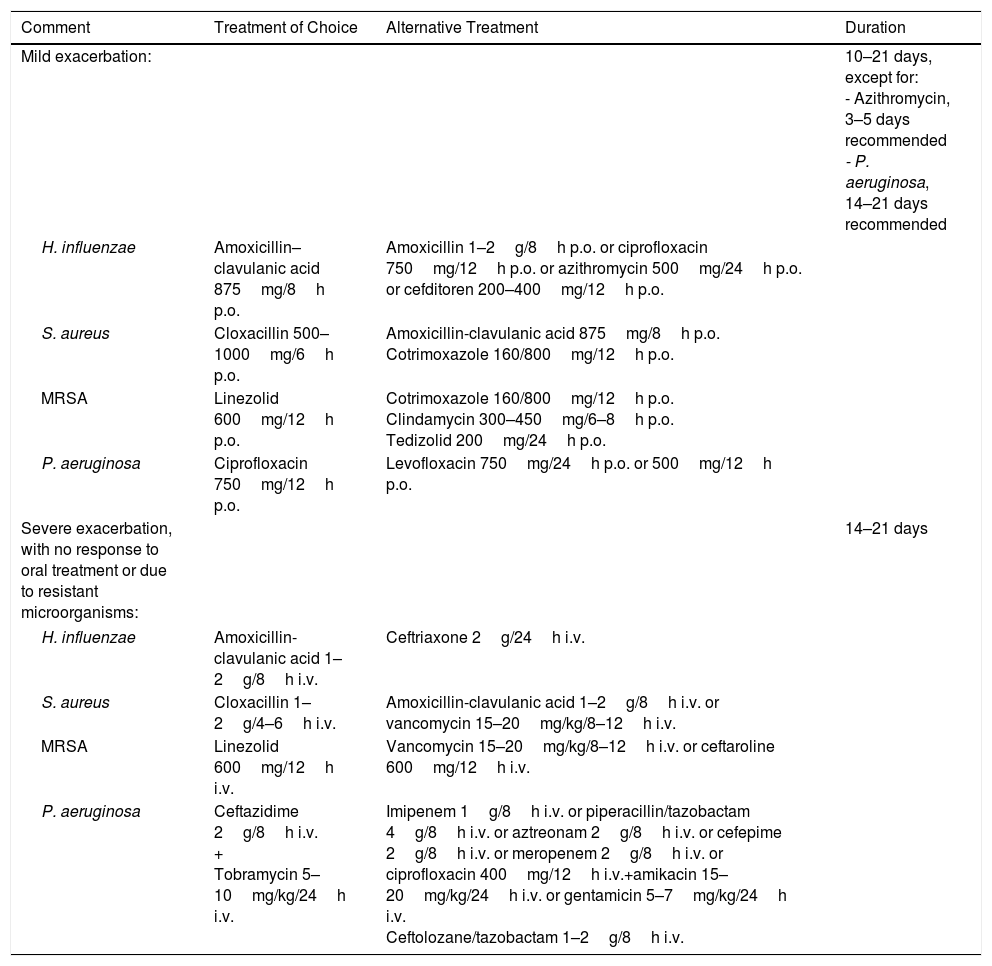
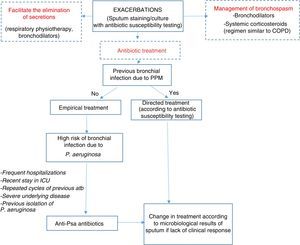
Therapeutic AlgorithmEmpirical antibiotic treatment should be started based on the previous culture, if available. Table 8 shows the different therapeutic options depending on the microorganism and severity of the exacerbation. The sputum sample for microbiological study in exacerbations should be taken prior to starting treatment in order to improve yield (Fig. 1).
Antibiotic Treatment in Exacerbations.
| Comment | Treatment of Choice | Alternative Treatment | Duration |
|---|---|---|---|
| Mild exacerbation: | 10–21 days, except for: - Azithromycin, 3–5 days recommended - P. aeruginosa, 14–21 days recommended | ||
| H. influenzae | Amoxicillin–clavulanic acid 875mg/8h p.o. | Amoxicillin 1–2g/8h p.o. or ciprofloxacin 750mg/12h p.o. or azithromycin 500mg/24h p.o. or cefditoren 200–400mg/12h p.o. | |
| S. aureus | Cloxacillin 500–1000mg/6h p.o. | Amoxicillin-clavulanic acid 875mg/8h p.o. Cotrimoxazole 160/800mg/12h p.o. | |
| MRSA | Linezolid 600mg/12h p.o. | Cotrimoxazole 160/800mg/12h p.o. Clindamycin 300–450mg/6–8h p.o. Tedizolid 200mg/24h p.o. | |
| P. aeruginosa | Ciprofloxacin 750mg/12h p.o. | Levofloxacin 750mg/24h p.o. or 500mg/12h p.o. | |
| Severe exacerbation, with no response to oral treatment or due to resistant microorganisms: | 14–21 days | ||
| H. influenzae | Amoxicillin-clavulanic acid 1–2g/8h i.v. | Ceftriaxone 2g/24h i.v. | |
| S. aureus | Cloxacillin 1–2g/4–6h i.v. | Amoxicillin-clavulanic acid 1–2g/8h i.v. or vancomycin 15–20mg/kg/8–12h i.v. | |
| MRSA | Linezolid 600mg/12h i.v. | Vancomycin 15–20mg/kg/8–12h i.v. or ceftaroline 600mg/12h i.v. | |
| P. aeruginosa | Ceftazidime 2g/8h i.v. + Tobramycin 5–10mg/kg/24h i.v. | Imipenem 1g/8h i.v. or piperacillin/tazobactam 4g/8h i.v. or aztreonam 2g/8h i.v. or cefepime 2g/8h i.v. or meropenem 2g/8h i.v. or ciprofloxacin 400mg/12h i.v.+amikacin 15–20mg/kg/24h i.v. or gentamicin 5–7mg/kg/24h i.v. Ceftolozane/tazobactam 1–2g/8h i.v. | |
i.v.: intravenous; MRSA: methycillin-resistant Staphylococcus aureus; p.o.: orally.
In the case of empirical treatment, microorganisms previously isolated should be treated and then treatment modified according to the sputum culture.
Algorithm for the management of exacerbations. antiPsa: anti-pseudomonas; Atb: antibiotic; COPD: chronic obstructive pulmonary disease; ICU: Intensive Care Unit; PPM: potentially pathogenic microorganisms. For choice of antibiotic and posology, see Table 8.
BE is a disease that requires multidisciplinary management, so all care levels should be involved.
Primary care: clinical suspicion, differential diagnosis with other airway diseases, referral to specialists for diagnosis and etiological study, prioritization of referral, monitoring of the non-severe, stable patient, and monitoring of mild–moderate exacerbations as well as mild treatment side effects.
Nursing: control of treatment adherence, assessment of tolerance, education in the use of inhalers, nebulizers and intravenous therapy, respiratory physiotherapy, as well as cleanliness of nebulization systems and performing spirometries.
Specialized clinics/units (not available in all hospitals): patients who require a diagnosis or specialized treatment (chronic bronchial infection [especially P. aeruginosa infection]), as well as those who present numerous exacerbations, an E-FACED score=6–9 points or Bronchiectasis Severity Index (BSI) score >9 points, some etiologies such as primary immunodeficiencies or ciliary dyskinesias, as well as those with poor control or disease progression should be referred to these clinics.
Hospital-in-the-home unit or home care teams: management of the patient with home intravenous treatment, patients in a terminal or advanced stage or patients with difficulty getting to hospital.
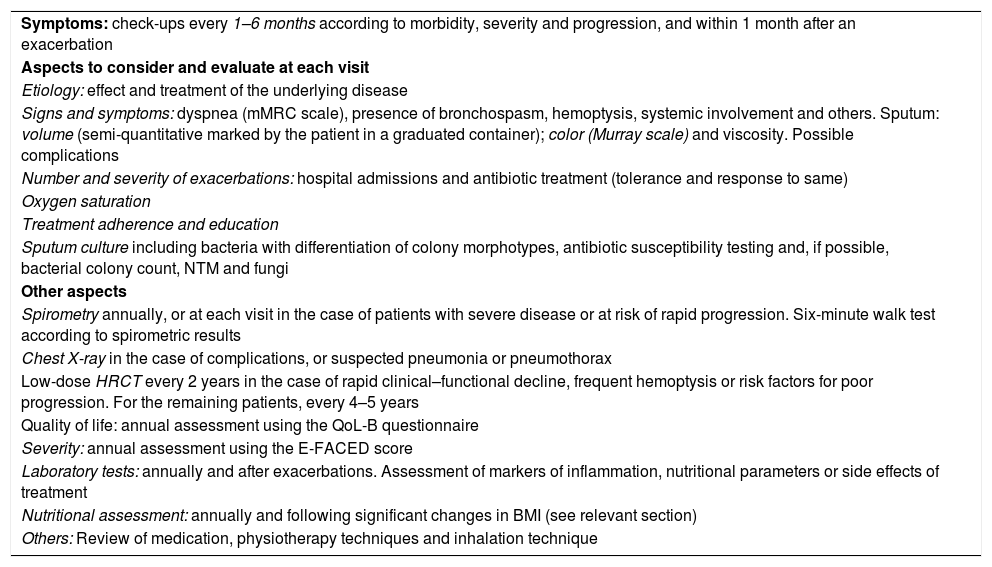
Follow-UpThe frequency and intensity of follow-up of patients with BE depends on their initial severity, disease progression, their follow-up center, and the healthcare resources available. Table 9 shows the recommended timing of visits and tests. In general, patients who are monitored in specialized BE clinics or units should been seen at least once every 6–12 months. More severe or unstable patients are advised to attend once every 1–3 months, with clinical and microbiological study performed at all visits. After an acute exacerbation, clinical assessment should be performed within the first month.
Assessment of Severity and Follow-Up.
| Symptoms: check-ups every 1–6 months according to morbidity, severity and progression, and within 1 month after an exacerbation |
| Aspects to consider and evaluate at each visit |
| Etiology: effect and treatment of the underlying disease |
| Signs and symptoms: dyspnea (mMRC scale), presence of bronchospasm, hemoptysis, systemic involvement and others. Sputum: volume (semi-quantitative marked by the patient in a graduated container); color (Murray scale) and viscosity. Possible complications |
| Number and severity of exacerbations: hospital admissions and antibiotic treatment (tolerance and response to same) |
| Oxygen saturation |
| Treatment adherence and education |
| Sputum culture including bacteria with differentiation of colony morphotypes, antibiotic susceptibility testing and, if possible, bacterial colony count, NTM and fungi |
| Other aspects |
| Spirometry annually, or at each visit in the case of patients with severe disease or at risk of rapid progression. Six-minute walk test according to spirometric results |
| Chest X-ray in the case of complications, or suspected pneumonia or pneumothorax |
| Low-dose HRCT every 2 years in the case of rapid clinical–functional decline, frequent hemoptysis or risk factors for poor progression. For the remaining patients, every 4–5 years |
| Quality of life: annual assessment using the QoL-B questionnaire |
| Severity: annual assessment using the E-FACED score |
| Laboratory tests: annually and after exacerbations. Assessment of markers of inflammation, nutritional parameters or side effects of treatment |
| Nutritional assessment: annually and following significant changes in BMI (see relevant section) |
| Others: Review of medication, physiotherapy techniques and inhalation technique |
BMI: body mass index; HRCT: high-resolution computed tomography; mMRC: modified Medical Research Council scale; NTM: non-tuberculous mycobacteria; QoL-B: Quality of Life bronchiectasis questionnaire.
Murray et al. Eur Resp J. 2009;34:361–4.
Miguel Ángel Martínez has participated in training sessions sponsored by Gilead, Novartis, Glaxo, Praxis, Teva and Zambon. He has also been the principal investigator in projects funded by Praxis and Zambon, and has participated in meetings analyzing clinical trial outcomes organized by Bayer and Grifols.
Luis Máiz has participated in training sessions sponsored by Gilead, Novartis, Zambon and Praxis.
Casilda Olveira has participated in training activities or expert committees sponsored by Gilead, Praxis, Novartis, Teva and Zambon.
Rosa Maria Girón Moreno has participated in training sessions sponsored by Gilead, Teva and Zambon.
Marina Blanco Aparicio has participated in training sessions sponsored by Zambon and Praxis Pharmaceutical, and has been principal investigator in a clinical trial on inhaled antibiotic therapy sponsored by Bayer.
David de la Rosa has participated in training sessions sponsored by Praxis, Zambon and Teva.
Rafael Cantón has participated in training sessions sponsored by Gilead, MSD, Novartis and Zambon. He has also been the principal investigator in projects funded by AZ and MSD, and has participated in meetings analyzing clinical trial outcomes organized by Bayer.
Montserrat Vendrell has participated in training sessions sponsored by Praxis, Zambon, Novartis and Chiesi. She has been principal investigator in projects funded by Praxis, Zambon and Chiesi. She has participated in meeting held by Grifols and Raptor pharmaceuticals.
Eva Polverino has been principal investigator in clinical trials sponsored by Bayer, Grifols, Insmed and Chiesi; she has participated in meetings analyzing clinical trial outcomes organized by Bayer and Insmed; she has participated in training sessions sponsored by Zambon.
Javier de Gracia has participated in training sessions sponsored by Gilead, Novartis and Zambon. He has also been principal investigator in projects funded by Bayer and Gilead.
Concepción Prados has participated in meetings organized by Gilead, Praxis, Zambon, Teva and Vertex.
David Rigau. Iberoamerican Cochrane Center. Barcelona. Spain. DRigau@santpau.cat.
Gabriel Olveira. Endocrinology and Nutrition Service, Nutrition Unit, Regional University Málaga Hospital, CIBERDEM, CIBER of Diabetes and Associated Metabolic Diseases (Instituto de Salud Carlos III), Madrid, Spain. e-mail: gabrielm.olveira.sspa@juntadeandalucia.es.
Radiology: M.a Isabel Marco Galve. Department of Radiology. Hospital de Alta Resolución de Benalmádena (E.P. Hospital Costa del Sol). Málaga. isabelmarcogalve@yahoo.es.
Physiotherapy and Rehabilitation: Marta López Martín. Department of Rehabilitation and Physical Medicine. Hospital Universitario de la Princesa. Madrid. marta.lopez.martin@salud.madrid.org.
Jordi Vilaró Casamitjana. Blanquerna Faculty of Health Sciences, Research Group on Health, Physical Activity and Sport, Ramon Llull University, Barcelona, Spain. Jordi.gestos@gmail.com.**
Please cite this article as: Martínez-García MÁ, Máiz L, Olveira C, Girón RM, de la Rosa D, Blanco M, et al. Normativa sobre el tratamiento de las bronquiectasias en el adulto. Arch Bronconeumol. 2018;54:88–98.
Annex 4 online contains an extensive set of literature references for each of the points discussed in these guidelines.