In 2008, the Spanish Society of Pulmonology (SEPAR) published the first guidelines in the world on the diagnosis and treatment of bronchiectasis. Almost 10 years later, considerable scientific advances have been made in both the treatment and the evaluation and diagnosis of this disease, and the original guidelines have been updated to include the latest scientific knowledge on bronchiectasis. These new recommendations have been drafted following a strict methodological process designed to ensure the quality of content, and are linked to a large amount of online information that includes a wealth of references. These guidelines cover aspects ranging from a consensual definition of bronchiectasis to an evaluation of the natural course and prognosis of the disease. The topics of greatest interest and some new areas are addressed, including epidemiology and economic costs of bronchiectasis, pathophysiological aspects, the causes (placing particular emphasis on the relationship with other airway diseases such as chronic obstructive pulmonary disease and asthma), clinical and functional aspects, measurement of quality of life, radiological diagnosis and assessment, diagnostic algorithms, microbiological aspects (including the definition of key concepts, such as bacterial eradication or chronic bronchial infection), and the evaluation of severity and disease prognosis using recently published multidimensional tools.
En 2008 la Sociedad Española de Neumología y Cirugía Torácica (SEPAR) publicó las primeras normativas del mundo sobre el diagnóstico y tratamiento de las bronquiectasias. Tras casi una década muchos han sido los avances científicos en esta enfermedad, no solo en sus aspectos terapéuticos, sino también en su valoración y diagnóstico. Por ello, estas nuevas normativas sobre la valoración y diagnóstico de las bronquiectasias tratan de ofrecer al lector una actualización del conocimiento científico sobre las bronquiectasias basándose en un estricto procedimiento metodológico que asegura la calidad del contenido de las mismas, y en una amplia cantidad de información online que incluye abundante bibliografía. Estas normativas recogen desde una definición consensuada de bronquiectasias hasta la valoración de la historia natural y del pronóstico de la enfermedad. Se tratan los temas de mayor interés y algunos novedosos, como epidemiología y costes económicos de las bronquiectasias, aspectos fisiopatológicos, etiología (haciendo especial énfasis en la relación con otras enfermedades de la vía aérea como la enfermedad pulmonar obstructiva crónica y el asma), aspectos clínico-funcionales, medición de la calidad de vida, diagnóstico y valoración radiológica, algoritmo diagnóstico, aspectos microbiológicos (incluyendo la definición consensuada de conceptos clave como el de erradicación bacteriana o infección bronquial crónica), así como la valoración de la gravedad y el pronóstico de la enfermedad mediante el uso de las nuevas herramientas multidimensionales publicadas.
Non-cystic fibrosis (CF) bronchiectasis (BE) is the third most common chronic inflammatory disease of the airways after asthma and chronic obstructive pulmonary disease (COPD), and is closely related to both. In 2008, the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) became the first scientific society to establish guidelines on the diagnosis and treatment of this disease, including CF.1 More than 8 years later, the scientific evidence on BE has become clearer on a number of major issues, and the findings of recent studies have compelled us to publish these new guidelines, which, in order to provide the reader with more specific information, will focus solely on BE in adults. This first section will be devoted to the assessment and diagnosis of BE. The guidelines have been prepared with the advice of an expert in methodology. A Delphi system was used to create the list of topics, prioritizing the clinical questions (Annex 1, Methodology). Key clinical questions were structured according to the Patient-Intervention-Comparison-Outcome (PICO) system, and appear as an annex at the end of the manuscript (Annex 3).
Finally, the certainty of the evidence and the strength of the recommendations were established following the Grades of Recommendation Assessment, Development and Evaluation (GRADE) system (Annex 4 online. This annex contains an extensive set of literature references for each of the points discussed in these guidelines).
DefinitionBronchiectasis is a chronic inflammatory bronchial disease with irreversible dilatation of the bronchial lumen that can be caused by different etiologies. Clinically, it usually presents with chronic cough and expectoration, as well as recurrent infectious exacerbations. It can cause chronic bronchial infection and a progressive decline in lung function, all of which can lead to a deterioration in quality of life and increased morbidity and mortality. Traction BE, secondary to another lung disease (interstitial or emphysematous), is not considered in the present guidelines.
Epidemiology and CostsAlthough the actual prevalence of BE is unknown, it is estimated to be between 42 and 566 cases per 100000 population (higher in women and the elderly), although it is recognized as being significantly underdiagnosed. These figures confirm that it is not a rare disease, as it exceeds the 5 cases per 10000 population established in the definition of orphan disease in Europe. We are currently witnessing a major increase in the number of cases diagnosed with BE, possibly due to the growing longevity of the population, the chronic nature of underlying diseases, the recently observed association between BE and other highly prevalent entities (such as asthma or COPD) and, above all, the widespread use of imaging techniques to confirm diagnosis (chest high-resolution computed tomography [HRCT]). The cost of BE is high (the average cost of annual treatment in Spain is estimated to be close to €4700), and is greater the more severe the disease (around €10000 annually in severe cases), if there is coexisting COPD, a higher number of exacerbations, and when there is chronic bronchial Pseudomonas aeruginosa infection. Most of the cost is due to exacerbations and inhaled antibiotic treatment in severe BE. Cost-effectiveness studies of currently available treatments for BE are needed.2,3
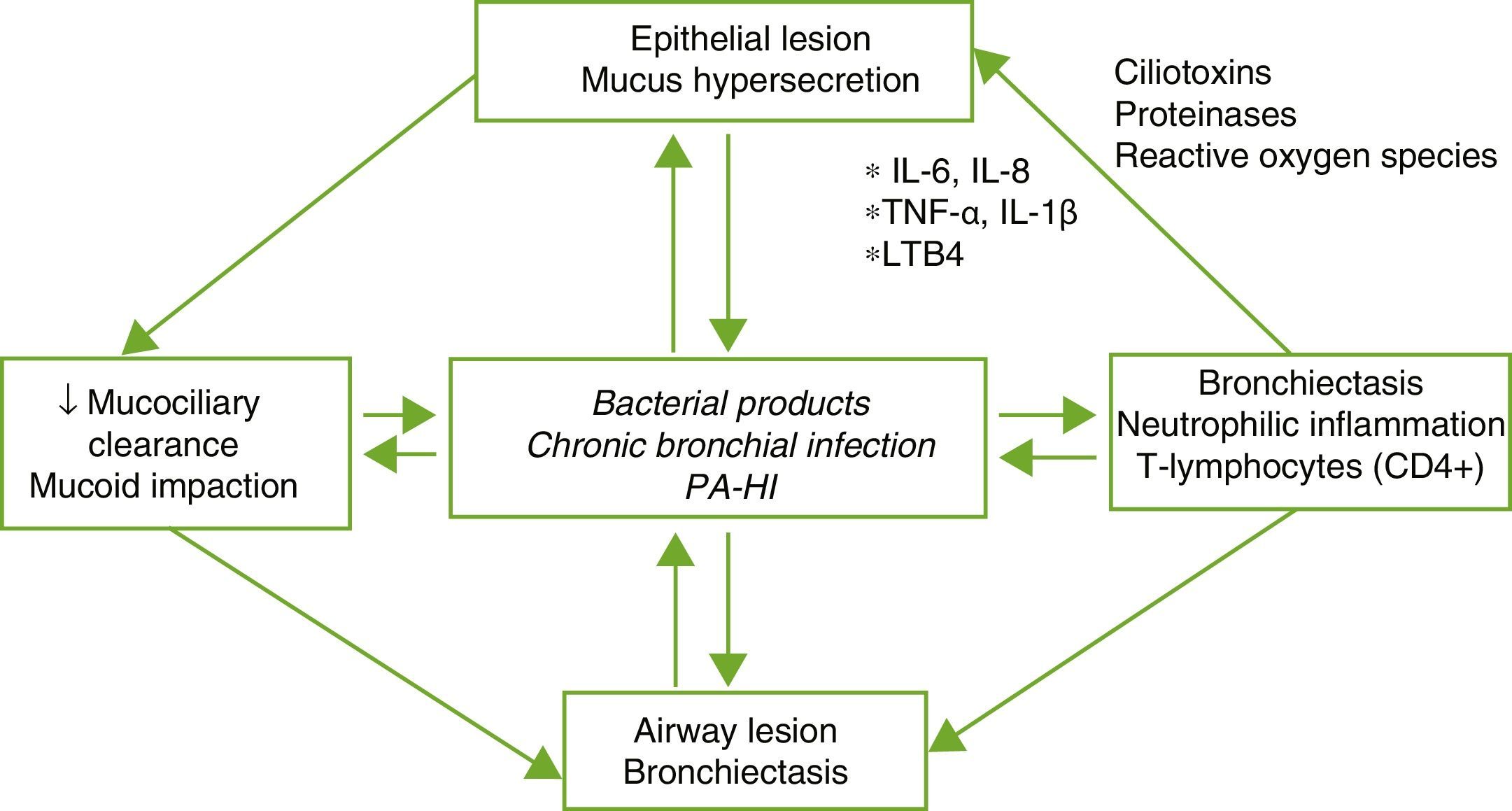
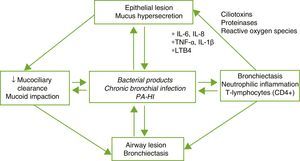
PhysiopathologyBE is the result of a complex vicious circle consisting of lesion of the mucociliary system, inflammation, infection and airway repair, which differ according to the specific etiology that triggers the initial abnormality. Damage to the mucociliary system makes it difficult to eliminate secretions and facilitates bacterial growth and bronchial inflammation, with the latter 2 being responsible for the bronchial structural damage and perpetuation of the vicious pathogenic circle (Fig. 1). An imbalance between pro- and anti-inflammatory products, and persistent infection and inflammation despite the immune response and treatment, could play an important role in disease progression. Inflammation of the airways has a neutrophilic profile. A high percentage of patients with BE also present systemic inflammation in the stable phase of the disease, which has been related with more severe forms.4,5
Pathogenesis of bronchiectasis. HI: Haemophilus influenzae; IL: interleukin; LTB4: leukotriene B4; PA: Pseudomonas aeruginosa; TNF: tumor necrosis factor.
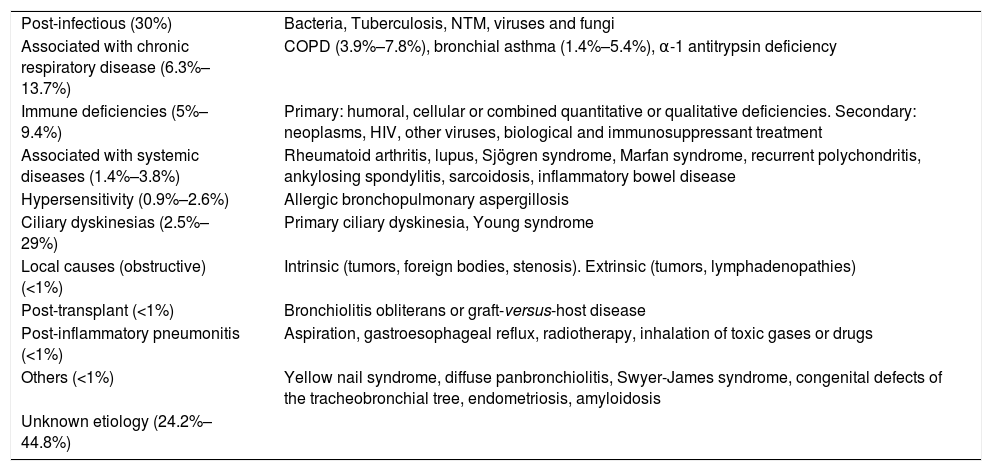
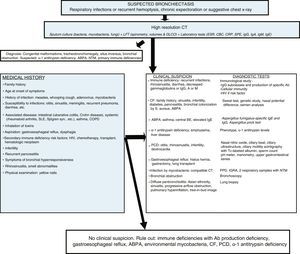
BE can be caused by a number of different etiologies, both pulmonary and systemic. The relative frequency of these etiologies depends on the geographical area in which it is studied, the characteristics of the patient and the clinic attended (general or specialized clinics). The post-infection forms are the most common in most series (Table 1). BEs of unknown origin (or idiopathic) are considered to be those in which the cause is unknown despite a comprehensive etiological study (Fig. 2, diagnostic algorithm), and could account for between 25% and 45% of cases, according to the series.6–8 It is believed that a significant percentage of these BE could be due to selective immune deficiencies, gastroesophageal reflux, infections not reported by the patient or other airway diseases such as COPD or asthma.
Etiology and Diseases Associated With Bronchiectasis.
| Post-infectious (30%) | Bacteria, Tuberculosis, NTM, viruses and fungi |
| Associated with chronic respiratory disease (6.3%–13.7%) | COPD (3.9%–7.8%), bronchial asthma (1.4%–5.4%), α-1 antitrypsin deficiency |
| Immune deficiencies (5%–9.4%) | Primary: humoral, cellular or combined quantitative or qualitative deficiencies. Secondary: neoplasms, HIV, other viruses, biological and immunosuppressant treatment |
| Associated with systemic diseases (1.4%–3.8%) | Rheumatoid arthritis, lupus, Sjögren syndrome, Marfan syndrome, recurrent polychondritis, ankylosing spondylitis, sarcoidosis, inflammatory bowel disease |
| Hypersensitivity (0.9%–2.6%) | Allergic bronchopulmonary aspergillosis |
| Ciliary dyskinesias (2.5%–29%) | Primary ciliary dyskinesia, Young syndrome |
| Local causes (obstructive) (<1%) | Intrinsic (tumors, foreign bodies, stenosis). Extrinsic (tumors, lymphadenopathies) |
| Post-transplant (<1%) | Bronchiolitis obliterans or graft-versus-host disease |
| Post-inflammatory pneumonitis (<1%) | Aspiration, gastroesophageal reflux, radiotherapy, inhalation of toxic gases or drugs |
| Others (<1%) | Yellow nail syndrome, diffuse panbronchiolitis, Swyer-James syndrome, congenital defects of the tracheobronchial tree, endometriosis, amyloidosis |
| Unknown etiology (24.2%–44.8%) |
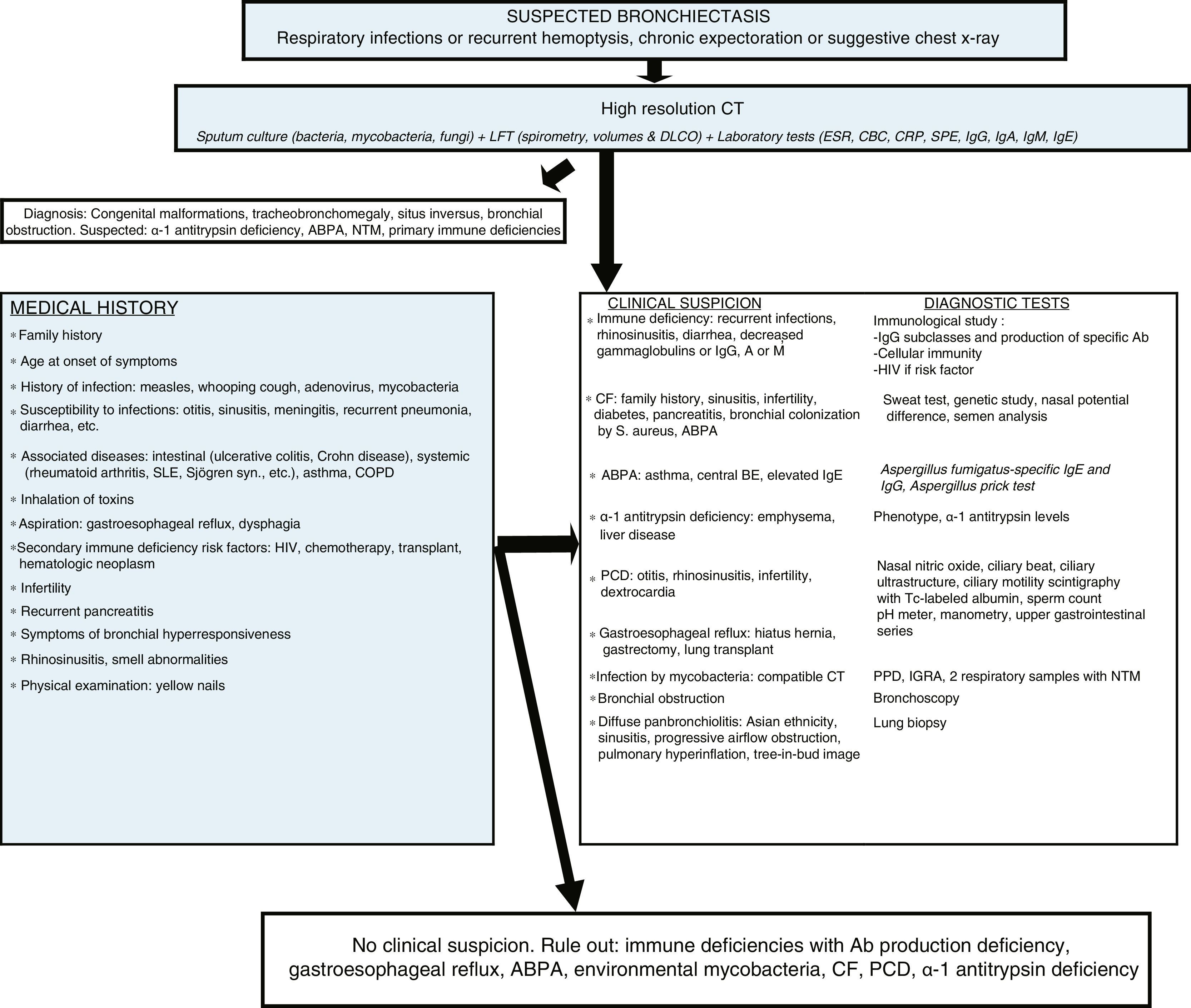
Diagnostic algorithm. Ab: antibodies; ABPA: allergic bronchopulmonary aspergillosis; BE: bronchiectasis; CBC: complete blood count; CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; CRP: C-reactive protein; CT: computed tomography; PCD: primary ciliary dyskinesia; DLCO: diffusing capacity of the lungs for carbon monoxide; ESR: erythrocyte sedimentation rate; FEV1: forced expiratory volume in the first second; HIV: human immunodeficiency virus; Ig: immunoglobulin; IGRA: interferon gamma release assay; LFT: lung function tests; NTM: non-tuberculous mycobacteria; PPD: purified protein derivative skin test; S: Staphylococcus; SLE: systemic lupus erythematosus; SPE: serum protein electrophoresis; Syn: syndrome; Tc: technetium; UGI: upper gastrointestinal.
Between 30% and 50% of patients with severe COPD have BE, and its prevalence increases with the severity of the COPD, while 5%–10% of patients with BE have associated COPD.6–8 Patients with COPD and BE make up a clinical group or phenotype with its own characteristics (greater production and purulence of sputum, more dyspnea and a higher number of exacerbations), worse prognosis and possible therapeutic implications. HRCT should be performed to rule out the presence of BE in patients with moderate or severe COPD with multiple exacerbations, and/or repeated isolation of potentially pathogenic microorganisms (PPM) in respiratory samples (or Pseudomonas aeruginosa only) in a clinically stable phase. Although a causality relationship between both entities has not been studied, it is biologically plausible that this is the case.9,10
Relationship Between Bronchiectasis and AsthmaThe prevalence of BE in severe or uncontrolled asthma is 20%–30%. The possible impact of BE on asthma is unknown, although it usually appears in severe, uncontrolled or neutrophilic asthma.11 Similarly, the pathophysiological mechanism of these entities and the existence of a causality relationship are unknown. In patients with central BE who present with symptoms suggestive of asthma, allergic bronchopulmonary aspergillosis (ABPA) should be ruled out.
DiagnosisClinical AspectsForms of PresentationPatients with BE usually present clinically with chronic cough (41%–100%), chronic (46%–76%) or intermittent expectoration (20%–38%), and repeated respiratory infections, but can remain asymptomatic between these episodes. Other symptoms that often present are dyspnea, hemoptysis of varying intensity, intermittent chest pain and fatigue. Acropachy is rare and usually appears in advanced stages. Sinusitis is common, especially in primary ciliary dyskinesia and in primary immune deficiencies.
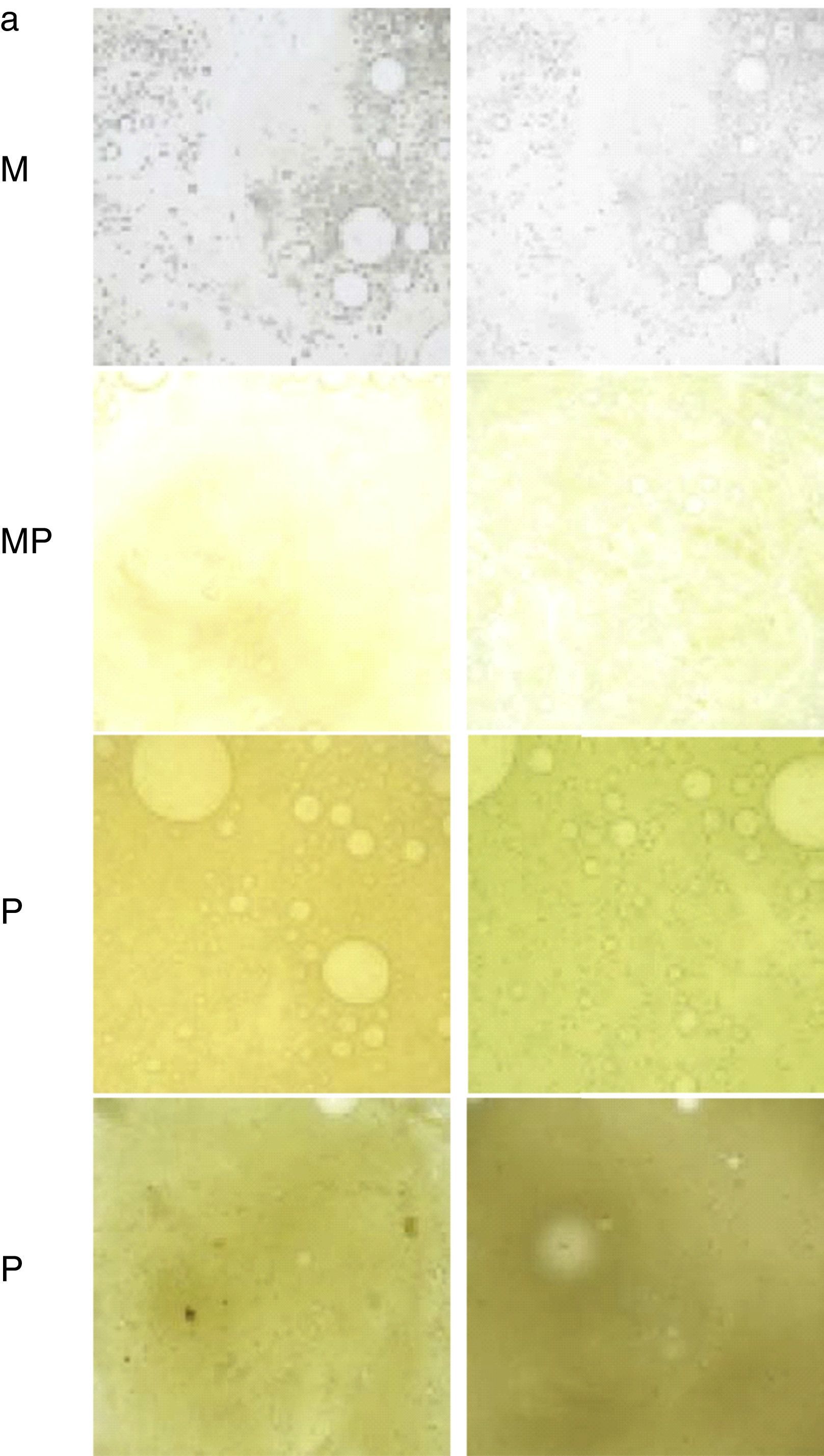
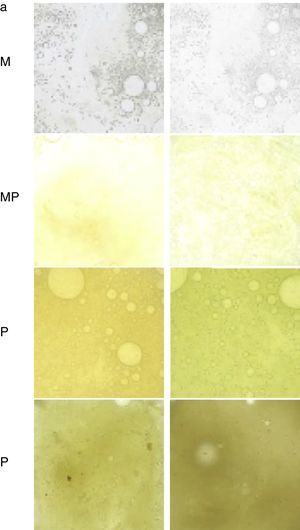
AnamnesisThis should include the most common symptoms of BE mentioned in the previous section, as well as tests aimed at identifying a specific cause (Fig. 2, diagnostic algorithm). It is useful to quantify the daily volume (semi-quantitatively, marked by the patient in a graduated container) and color of the sputum (Murray scale, Fig. 3).12
Table to assess the color of sputum from least to most purulent. M: mucous; MP: mucopurulent; P: purulent.
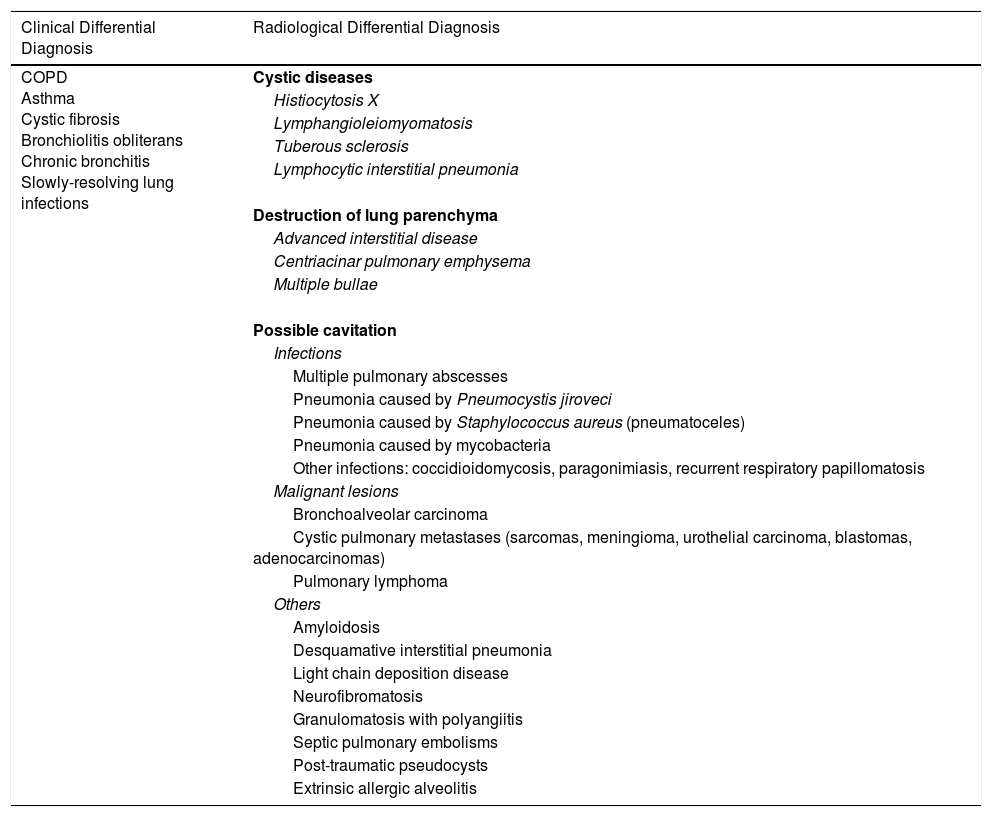
Other chronic respiratory diseases with similar symptoms or diffuse cystic lung diseases, or diseases that may present radiologically with cavitation should be considered in the differential diagnosis of BE (Table 2).
Respiratory Diseases Included in Clinical or Radiological Differential Diagnosis With Bronchiectasis.
| Clinical Differential Diagnosis | Radiological Differential Diagnosis |
|---|---|
| COPD Asthma Cystic fibrosis Bronchiolitis obliterans Chronic bronchitis Slowly-resolving lung infections | Cystic diseases |
| Histiocytosis X | |
| Lymphangioleiomyomatosis | |
| Tuberous sclerosis | |
| Lymphocytic interstitial pneumonia | |
| Destruction of lung parenchyma | |
| Advanced interstitial disease | |
| Centriacinar pulmonary emphysema | |
| Multiple bullae | |
| Possible cavitation | |
| Infections | |
| Multiple pulmonary abscesses | |
| Pneumonia caused by Pneumocystis jiroveci | |
| Pneumonia caused by Staphylococcus aureus (pneumatoceles) | |
| Pneumonia caused by mycobacteria | |
| Other infections: coccidioidomycosis, paragonimiasis, recurrent respiratory papillomatosis | |
| Malignant lesions | |
| Bronchoalveolar carcinoma | |
| Cystic pulmonary metastases (sarcomas, meningioma, urothelial carcinoma, blastomas, adenocarcinomas) | |
| Pulmonary lymphoma | |
| Others | |
| Amyloidosis | |
| Desquamative interstitial pneumonia | |
| Light chain deposition disease | |
| Neurofibromatosis | |
| Granulomatosis with polyangiitis | |
| Septic pulmonary embolisms | |
| Post-traumatic pseudocysts | |
| Extrinsic allergic alveolitis |
The most common functional abnormality in BE is chronic persistent airflow obstruction (with normal or slightly reduced forced vital capacity), more marked in smokers or COPD patients. Mixed patterns can appear in the post-tuberculous, fibrotic or destructive forms, although a pure restrictive pattern is rare. A slight decrease may be observed in the diffusing capacity of the lungs for carbon dioxide (DLCO). Bronchial hyperresponsiveness (BHR) has been observed in 30%–69% of cases.
Quality of LifePatients with BE have poorer quality of life scores than the general population. This deterioration has been related to a larger extent with age, chronic bronchial P. aeruginosa infection, grade of dyspnea, number of exacerbations, poorer lung function, presence of BHR, greater structural damage, chronic bronchorrhea, presence of respiratory failure and symptoms of depression and anxiety. The only questionnaires designed specifically for use in BE are the Quality of Life-Bronchiectasis13 questionnaire and the recently published Bronchiectasis Health Questionnaire. Other validated questionnaires are: St. George's Respiratory Questionnaire14 and the Leicester Cough Questionnaire,15 the latter for the specific assessment of the impact of cough. The Quality of Life-Bronchiectasis questionnaire is useful to assess the patient's perception of severity on an annual basis (http://www.psy.miami.edu/qol_b/qol_measures01.phtml).
Analytical AspectsSome systemic inflammatory markers, such as the peripheral neutrophil count, C-reactive protein (CRP) level and erythrocyte sedimentation rate are associated with accelerated loss of lung function and greater radiological extension; determination of CRP has been shown to be most useful.
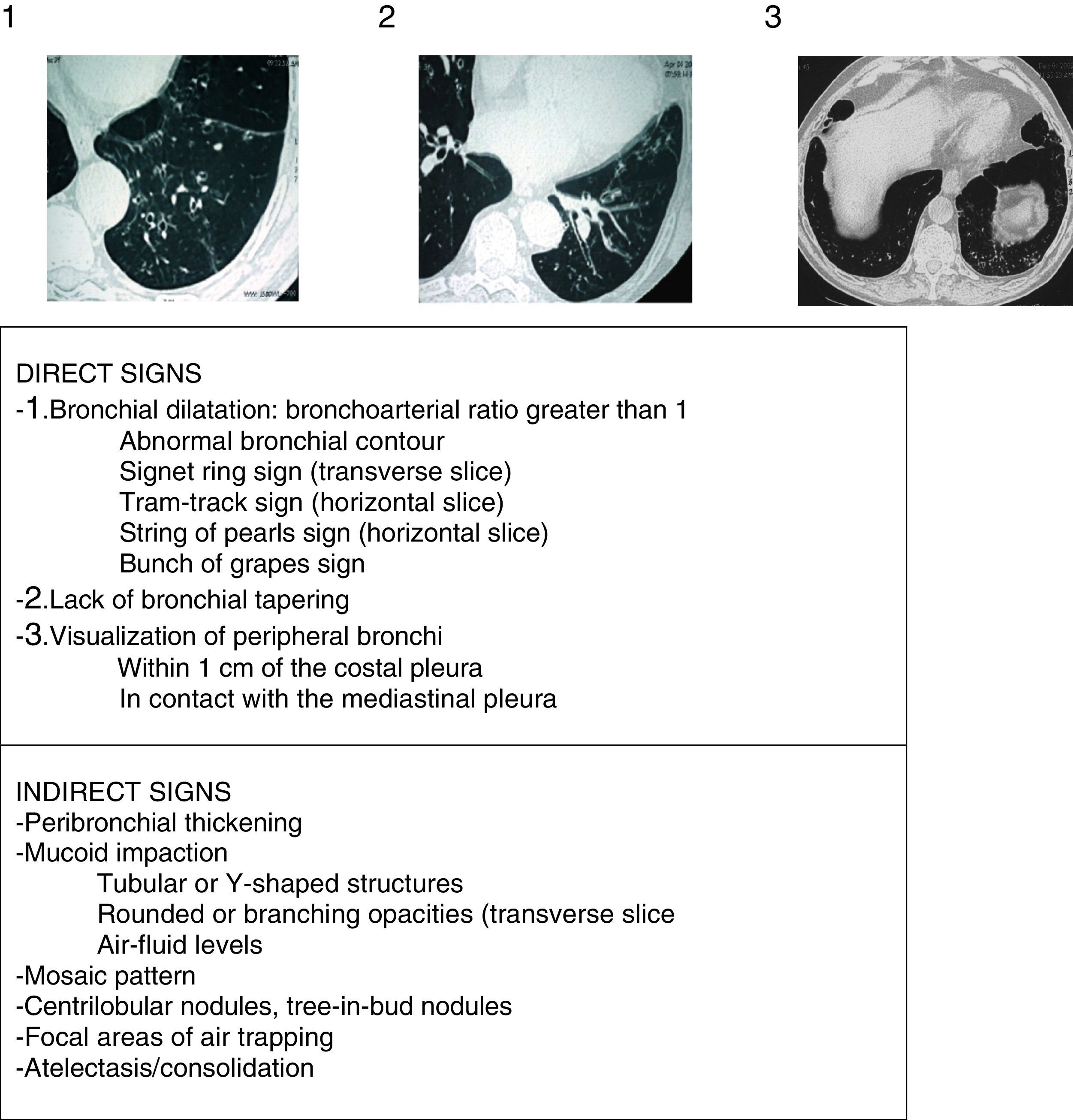
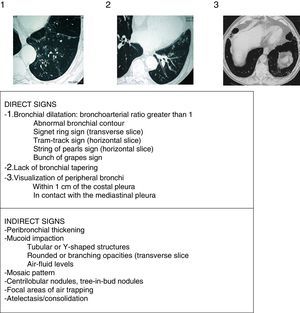
Radiological AspectsChest radiograph shows low sensitivity and specificity for the diagnosis of BE. It should be performed when complications (such as pneumonia, pneumothorax or atelectasis) are suspected. HRCT is currently the gold standard for both diagnosis and to assess disease morphology, extent and progression (strong recommendation, high quality evidence). It also helps in therapeutic decision-making and the diagnosis of concomitant findings. Low-dose (<1mSv) volumetric acquisition protocols without contrast are commonly used, with a high resolution reconstruction algorithm with 1–1.25mm slices every 10mm in maximum inspiration. The expiration images usually help to assess the presence of air trapping, bronchomalacia and small airway abnormalities. The criteria described by Naidich et al. (Fig. 4)16 are recommended for the radiological diagnosis of BE. The cardinal sign is the presentation of a bronchial dilatation, taking the diameter of the adjacent bronchial artery as a reference, although up to 20% of healthy elderly individuals may present this radiological criterion. In some cases, HRCT can reveal the etiology (Fig. 2). Diffuse BE suggests an underlying systemic problem, those due to tuberculosis predominate in upper fields and those secondary to ABPA are usually central. The presence of associated multiple small nodules, predominantly in the lingula and middle lobe, suggests non-tuberculous mycobacteria (NTM) infection.
Radiological signs of bronchiectasis (images above the table of the 3 principle criteria or direct signs of Naidich et al.).
In all patients, regardless of clinical suspicion of the cause that precipitates the BE, a detailed clinical history should be taken, and a microbiological study including bacteria, mycobacteria and fungi together with laboratory tests that include serum protein electrophoresis (SPE) and IgG, IgA, IgM and IgE immunoglobulin (Ig) levels should be performed. The results of these tests can shed light on most causes, and rationally indicate the diagnostic tests needed for confirmation. If an etiological diagnosis cannot be reached, causes requiring specific management or treatment must always be ruled out. IgG subclass deficiency should be confirmed with an antibody production study; this can only be omitted in cases with very low IgG2 levels.17 The study of autoimmune diseases and HIV infection should only be done in the case of clinical suspicion, because BE is rarely the first manifestation. A post-infectious cause should only be considered when BE symptoms present after an episode of acute respiratory infection or pneumonia, provided other causes have been previously excluded. The diagnosis of immune deficiencies with defective antibody production, CF and primary ciliary dyskinesia should be confirmed in specialized centers.
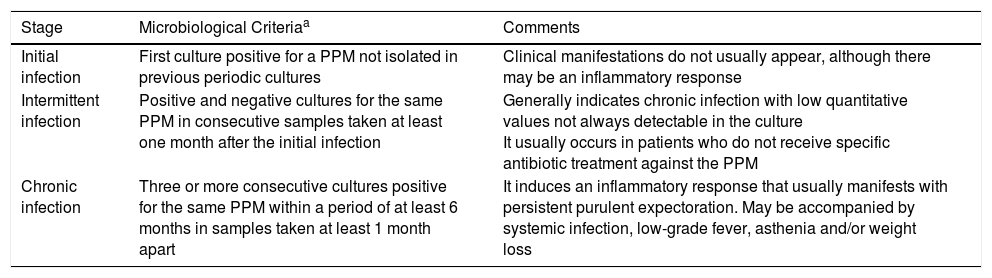
Microbiological AspectsColonization and Infection in BronchiectasisIt is preferable to use the term “pathogenic colonization” which is usually expressed as “bronchial infection” and not as “bronchial colonization”. This is a “passive pathogenesis” model caused by the growth of microorganisms on the surface of the respiratory mucosa without invading the adjacent tissues and which causes a local inflammatory effect. Different stages in the infection can be distinguished in BE, which are important in clinical management and antimicrobial treatment (Table 3).
Stages of Bronchial Infection (Pathogenic Colonization) in Bronchiectasis.
| Stage | Microbiological Criteriaa | Comments |
|---|---|---|
| Initial infection | First culture positive for a PPM not isolated in previous periodic cultures | Clinical manifestations do not usually appear, although there may be an inflammatory response |
| Intermittent infection | Positive and negative cultures for the same PPM in consecutive samples taken at least one month after the initial infection | Generally indicates chronic infection with low quantitative values not always detectable in the culture It usually occurs in patients who do not receive specific antibiotic treatment against the PPM |
| Chronic infection | Three or more consecutive cultures positive for the same PPM within a period of at least 6 months in samples taken at least 1 month apart | It induces an inflammatory response that usually manifests with persistent purulent expectoration. May be accompanied by systemic infection, low-grade fever, asthenia and/or weight loss |
PPM: potentially pathogenic microorganisms.
Eradication of a certain PPM is the absence of cultures positive for the microorganism in at least 3 sputum samples taken at least 1 month apart over a period of 6 months. Colony count in the culture is not common practice, but can help assess treatment efficacy.18
Potentially Pathogenic Microorganisms and MicrobiomeBronchial infection in BE is normally caused by so-called PPMs, which include non-typeable Haemophilus influenzae, P. aeruginosa, Streptococcus pneumoniae, Moraxella catharralis and Staphylococcus aureus, the latter being more common in CF; of these, P. aeruginosa has been associated with a worse prognosis. Recent improvements in microbiological methods have led to an increase in the isolation of enterobacteria, Gram-negative non-fermenting bacteria such as Achromobacter (Alcaligenes) xylosoxidans and Stenotrophomonas maltophilia, Nocardia spp., fungi (essentially Candida albicans and Aspergillus fumigatus, although also Scedosporium apiospermum) and NTM, some of which can have negative clinical and prognostic consequences for the patient (Mycobacterium abscessus). As yet, few studies have investigated methicillin-resistant S. aureus (MRSA) in BE, although its incidence may be rising.
Routine cultures for the detection of PPM, mycobacteria and fungi (yeasts and filamentous) are generally carried out in stable patients, and whenever there is an exacerbation, preferably before taking antibiotics. Furthermore, NTM culture should be performed in patients with fibronodular lesions in the radiological follow-up who do not respond to standard treatment and in whom clinical deterioration is noted, as well as in patients scheduled to start macrolide treatment.
There are still few studies on the microbiome associated with BE, and as yet insufficient scientific evidence to support routine studies of the respiratory microbiome or the search for anaerobic bacteria.19
Microbiological Diagnosis of the Bronchial InfectionThe microscopic examination of sputum should exclude contamination from the upper respiratory tract, so >25 leukocytes and <10 epithelial cells should be observed. Samples must be collected, transported and processed within 6h. If this is not possible, they should be kept for no longer than 24h at room temperature, preferably stored at 4°C rather than −20°C. For longer periods, they should be kept at −80°C. General differential and selective media should be included in the culture to increase yield. Routine bacterial counts are controversial due to the time needed to perform them, and the potential usefulness of the information obtained. Nevertheless, they should be used in the evaluation of new treatments, including combinations of antimicrobials, and to assess the eradication of PPMs. Different morphotypes of the same microorganism can appear in cultures and should be detected using specific antibiotic susceptibility testing (AST) in each case.
Although AST results are the gold standard in antimicrobial treatment, correlation between conventional in vitro sensitivity and in vivo response can be poor, especially with microorganisms that grow in biofilms, or while using inhaled antibiotics that reach very high concentrations in the bronchial mucosa. Therapeutic decisions, therefore, should be guided by the clinical response.
NTMs require culture with special media and must be expressly requested. An acid-fast stain (Ziehl-Neelsen or, preferably, auramine–rhodamine fluorescence staining) may be useful. If a positive stain is obtained, it should be confirmed and identified using molecular techniques, and AST should be carried out. Suspected Nocardia spp. infection should be discussed with the microbiologist to facilitate identification. Finally, the determination of anti-pseudomonas antibodies is unnecessary in bacterial cultures.
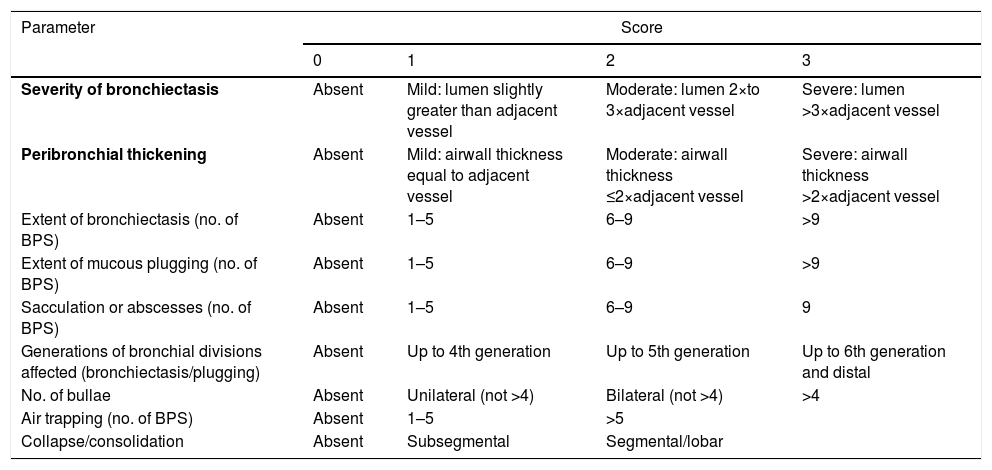
Severity and Prognostic FactorsRadiological ScoresOf the many radiological score systems, the modified Reiff score is recommended for its simplicity.20 This score is based on the diameter of the bronchial lumen/diameter of the adjacent vessel (0 points≤1; 1 point=1–2; 2 points=2–3; 3 points≥3) in each of the 6 lung lobes. The modified Bhalla score system (Table 4)21 is recommended if more extensive or detailed radiological information is needed. The correlation between both scores is very high.
Modified Bhalla Scoring System.
| Parameter | Score | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Severity of bronchiectasis | Absent | Mild: lumen slightly greater than adjacent vessel | Moderate: lumen 2×to 3×adjacent vessel | Severe: lumen >3×adjacent vessel |
| Peribronchial thickening | Absent | Mild: airwall thickness equal to adjacent vessel | Moderate: airwall thickness ≤2×adjacent vessel | Severe: airwall thickness >2×adjacent vessel |
| Extent of bronchiectasis (no. of BPS) | Absent | 1–5 | 6–9 | >9 |
| Extent of mucous plugging (no. of BPS) | Absent | 1–5 | 6–9 | >9 |
| Sacculation or abscesses (no. of BPS) | Absent | 1–5 | 6–9 | 9 |
| Generations of bronchial divisions affected (bronchiectasis/plugging) | Absent | Up to 4th generation | Up to 5th generation | Up to 6th generation and distal |
| No. of bullae | Absent | Unilateral (not >4) | Bilateral (not >4) | >4 |
| Air trapping (no. of BPS) | Absent | 1–5 | >5 | |
| Collapse/consolidation | Absent | Subsegmental | Segmental/lobar | |
The 3 parameters selected for the simplified scale are shown in bold.
In the original Bhalla scoring system, air trapping is replaced by the presence of emphysema, but with the same score.
BPS: bronchopulmonary segments.
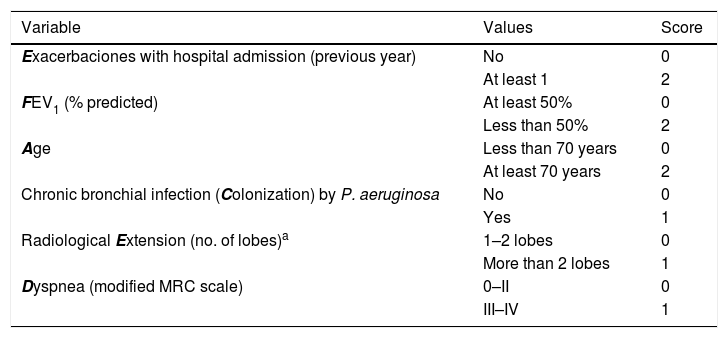
Two multidimensional scores are used to assess the prognosis and initial severity of BE: the FACED22 and the Bronchiectasis Severity Index (BSI),23 as well as a modification of the former (E-FACED),24 which also includes the number and severity of exacerbations in the previous year. For the initial clinical management and assessment of the patient, the E-FACED score is recommended for its simplicity (Table 5). The variables should be collected as close as possible to the time of diagnosis. Both the FACED and E-FACED have shown good prognostic capacity for mortality. E-FACED also presents a good prognostic capacity for the number and severity of exacerbations. The E-FACED should be obtained annually to assess clinical progression of the disease (strong recommendation, moderate quality evidence). Although the BSI (www.bronchiectasisseverity.com/) is more complex, it has also shown good prognostic capacity for quality of life and lung function decline.
E-FACED Multidimensional Scoring System.
| Variable | Values | Score |
|---|---|---|
| Exacerbaciones with hospital admission (previous year) | No | 0 |
| At least 1 | 2 | |
| FEV1 (% predicted) | At least 50% | 0 |
| Less than 50% | 2 | |
| Age | Less than 70 years | 0 |
| At least 70 years | 2 | |
| Chronic bronchial infection (Colonization) by P. aeruginosa | No | 0 |
| Yes | 1 | |
| Radiological Extension (no. of lobes)a | 1–2 lobes | 0 |
| More than 2 lobes | 1 | |
| Dyspnea (modified MRC scale) | 0–II | 0 |
| III–IV | 1 |
FEV1: forced expiratory volume in the first second; MRC: Medical Research Council.
Total range of growing severity: 0–9 points (E-FACED).
E-FACED classification of severity.
0–3 points: mild bronchiectasis.
4–6 points: moderate bronchiectasis.
7–9 points: severe bronchiectasis.
BE is an irreversible, chronic disease with variable progression. As the disease progresses, a greater number of exacerbations and hospital admissions, progressive airflow obstruction, chronic bronchial infection caused by P. aeruginosa and other multiresistant PPMs, progressive dyspnea, respiratory failure, cor pulmonale and death (especially due to respiratory exacerbations) usually appear. The presence of systemic inflammation, chronic bronchial P. aeruginosa infection and severe exacerbations have been associated with more rapid progression of BE.25
Conflict of InterestsMiguel Ángel Martínez has participated in training sessions sponsored by Gilead, Novartis, Glaxo, Praxis, Teva and Zambon. He has also been the principal investigator in projects funded by Praxis and Zambon, and has participated in meetings analyzing clinical trial outcomes organized by Bayer and Grifols.
Luis Máiz has participated in training sessions sponsored by Gilead, Novartis, Zambon and Praxis.
Casilda Olveira has participated in training activities or expert committees sponsored by Gilead, Praxis, Novartis, Teva and Zambon.
Rosa Maria Girón Moreno has participated in training sessions sponsored by Gilead, Teva and Zambon.
Marina Blanco Aparicio has participated in training sessions sponsored by Zambon and Praxis Pharmaceutical, and has been principal investigator in a clinical trial on inhaled antibiotic therapy sponsored by Bayer.
David de la Rosa has participated in training sessions sponsored by Praxis, Zambon and Teva.
Rafael Cantón has participated in training sessions sponsored by Gilead, MSD, Novartis and Zambon. He has also been the principal investigator in projects funded by AZ and MSD, and has participated in meetings analyzing clinical trial outcomes organized by Bayer.
Montserrat Vendrell has participated in training sessions sponsored by Praxis, Zambon, Novartis and Chiesi. She has been principal investigator in projects funded by Praxis, Zambon and Chiesi. She has participated in meeting held by Grifols and Raptor pharmaceuticals.
Eva Polverino has been principal investigator in clinical trials sponsored by Bayer, Grifols, Insmed and Chiesi; she has participated in meetings analyzing clinical trial outcomes organized by Bayer and Insmed; she has participated in training sessions sponsored by Zambon.
Javier de Gracia has participated in training sessions sponsored by Gilead, Novartis and Zambon. He has also been principal investigator in projects funded by Bayer and Gilead.
Concepción Prados has participated in meetings organized by Gilead, Praxis, Zambon, Teva and Vertex.
David Rigau. Iberoamerican Cochrane Centre, Barcelona, Spain. Email: DRigau@santpau.cat.
Gabriel Olveira. Endocrinology and Nutrition Service, Nutrition Unit, Regional University Malaga Hospital, CIBERDEM, CIBER of Diabetes and Associated Metabolic Diseases (Instituto de Salud Carlos III), Madrid, Spain. Madrid, Spain. Email: gabrielm.olveira.sspa@juntadeandalucia.es.
M.a Isabel Marco Galve. Department of Radiology. Hospital de Alta Resolución de Benalmádena (E.P. Hospital Costa del Sol). Malaga, Spain. Email: isabelmarcogalve@yahoo.es.
Marta López Martín. Department of Rehabilitation and Physical Medicine. Hospital Universitario de la Princesa. Madrid, Spain. Email: marta.lopez.martin@salud.madrid.org.
Jordi Vilaró Casamitjana. Blanquerna Faculty of Health Sciences, Research Group on Health, Physical Activity and Sport, Ramon Llull University, Barcelona, Spain. Email: Jordi.gestos@gmail.com.
Please cite this article as: Martínez-García MÁ, Máiz L, Olveira C, Girón RM, de la Rosa D, Blanco M, et al. Normativa sobre la valoración y el diagnóstico de las bronquiectasias en el adulto. Arch Bronconeumol. 2018;54:79–87.