Recognizing the clinical heterogeneity of COPD suggests a specific therapeutic approach directed by the so-called clinical phenotypes of the disease. The Spanish COPD Guidelines (GesEPOC) is an initiative of SEPAR, which, together with the scientific societies involved in COPD patient care, and the Spanish Patient Forum, has developed these new clinical practice guidelines. This present article describes the severity classification and the pharmacological treatment of stable COPD. GesEPOC identifies four clinical phenotypes with differential treatment: non-exacerbator, mixed COPD-asthma, exacerbator with emphysema and exacerbator with chronic bronchitis. Pharmacological treatment of COPD is based on bronchodilation in addition to other drugs depending on the clinical phenotype and severity. Severity is established by the BODE/BODEx multidimensional scales. Severity can also be approximated by assessing airflow obstruction, dyspnea, level of physical activity and history of exacerbations. GesEPOC is a new, more individualized approach to COPD treatment according to the clinical characteristics of the patients.
El reconocimiento de la heterogeneidad clínica de la EPOC sugiere un abordaje terapéutico específico dirigido por los llamados fenotipos clínicos de la enfermedad. La Guía Española de la EPOC (GesEPOC) es una iniciativa de la SEPAR que, conjuntamente con las sociedades científicas implicadas en la atención a pacientes con EPOC y el Foro Español de Pacientes, ha elaborado una nueva guía de práctica clínica. En el presente artículo se describe la clasificación de gravedad y el tratamiento farmacológico de la EPOC estable. GesEPOC identifica 4 fenotipos clínicos con tratamiento diferencial: no agudizador, mixto EPOC-asma, agudizador con enfisema y agudizador con bronquitis crónica. La base del tratamiento farmacológico de la EPOC es la broncodilatación, a la que se añaden diversos fármacos según el fenotipo clínico y la gravedad. La gravedad se establecerá por las escalas multidimensionales BODE/BODEx. Una aproximación a la gravedad también se puede conseguir a partir de la obstrucción al flujo aéreo, la disnea, el nivel de actividad física y la historia de agudizaciones. GesEPOC supone una nueva aproximación al tratamiento de la EPOC más individualizada según las características clínicas de los pacientes.
Chronic obstructive pulmonary disease (COPD) is essentially characterized by chronic airflow limitation that is irreversible and mainly associated with tobacco smoke. It is an underdiagnosed disease with high morbidity and mortality and is a very significant public health problem.1 COPD is a complex disease with a very heterogeneous clinical presentation. Within what we know as COPD, different phenotypes can be defined that have clinical, prognostic and therapeutic repercussions.2
Since 2009, the Spanish Ministry for Health and Social Policy, through the Plan for Quality of the National Health-care System (SNS) and the Strategy for COPD, has been working to identify a way to improve the care and quality of life of people with COPD and to reduce the incidence of the disease. The multidisciplinary team of the Strategy for COPD3 has promoted the development of guidelines with the participation of all its members. In this context, the Spanish COPD Guidelines (GesEPOC) were born, based on an initiative of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) which, together with the scientific societies involved in COPD patient care and the Spanish Patient Forum, has developed the reference recommendations for COPD management in Spain, known as GesEPOC.4
GesEPOC is a continuance of guidelines created by SEPAR, these being basically the 2008 SEPAR-ALAT guidelines5 and the 2010 SEPAR-SemFyC,6 including the latest advances made in diagnosis, treatment and severity classification. GesEPOC also compiles and adapts recommendations contained in the latest version of the Global Initiative for Obstructive Lung Diseases (GOLD) for the diagnosis and treatment of COPD.7
This article summarizes the most current aspects of the pharmacological treatment of stable COPD. Due to space restrictions, non-pharmacological aspects of the treatment will not be dealt with and nor will the methodological aspects of evaluating the evidence. All these aspects, together with the treatment of exacerbated COPD and patient care in the final stages of life, can be consulted in the complete version of the guidelines.
Clinical Phenotypes of COPDCOPD is a very heterogeneous disease and therefore it is not possible to categorize it by FEV1 alone. Phenotypes are used to refer to clinical forms of COPD patients.8,9 A group of international experts has defined COPD phenotypes as “those attributes of the disease that either alone or combined describe the differences between individuals with COPD regarding parameters that have clinical significance (symptoms, exacerbations, responses to treatment, progression rates of the disease, or death)”.8 Therefore, the phenotype should be able to classify patients into subgroups with prognostic value that can determine the best therapy to achieve better clinical results.10–12
The GesEPOC guidelines propose four phenotypes that determine differential treatment: (1) non-exacerbator, with emphysema or chronic bronchitis; (2) mixed COPD-asthma; (3) exacerbator with emphysema; (4) exacerbator with chronic bronchitis. Described below are the characteristics and definitions of the basic phenotypes, which, in the case of the exacerbator types, are combined with chronic bronchitis or emphysema in order to establish the definitive phenotype.
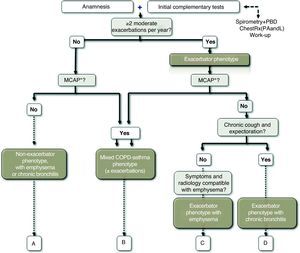
Definition of the Exacerbator PhenotypeThe exacerbator phenotype is defined as COPD patients who present with two or more moderate or severe exacerbations a year requiring treatment with systemic corticosteroids and/or antibiotics.13 In order to differentiate a new event from a previous therapeutic failure, exacerbations should be separated by at least 4 weeks from the resolution of the previous exacerbation or 6 weeks from the start of the exacerbation in cases that did not receive treatment.14
The identification of the exacerbator phenotype is based on the patient's medical history. It has also been demonstrated that diagnoses based on the declaration of the patient about his/her history of clinically relevant exacerbations are reliable.15 The exacerbator phenotype underlines the importance of asking about the history of exacerbations during the patient interview and to identify patients who can have an indication for anti-inflammatory treatment added to the bronchodilators. Frequent exacerbations can present in any of the three remaining phenotypes: emphysema, chronic bronchitis or mixed COPD-asthma.
Definition of Mixed COPD AsthmaThe mixed COPD phenotype is defined as an airflow obstruction that is not completely reversible accompanied by symptoms or signs of an increased reversibility of the obstruction.16,17 In other guidelines, these patients are described as “patients with COPD and prominent asthmatic component”18 or as asthma that complicates COPD.19
For the diagnosis of the mixed phenotype, a group of experts have agreed on some criteria that are presented in Table 1. For the diagnosis, 2 major criteria or 1 major and 2 minor criteria should be met.20 This classification is restrictive due to the lack of conclusive evidence between the relationship of the different criteria and the response to treatment in COPD. Prospective studies are required to validate these criteria.
Major and Minor Criteria for Establishing the Diagnosis of Mixed COPD Asthma Phenotype in COPD.20
| Major criteria |
| Very positive bronchodilator test (increase in FEV1>15% and >400mL) |
| Eosinophilia in sputum |
| Personal history of asthma |
| Minor criteria |
| High levels of total IgE |
| Personal history of atopy |
| Positive bronchodilator test on at least two occasions (increase of FEV1>12% and >200mL) |
The emphysema phenotype includes those COPD patients with clinical/radiological/functional diagnosis of emphysema, who present dyspnea and exercise intolerance as predominant symptoms. Patients with emphysema phenotype tend to present with a lower BMI. The diagnosis of the emphysema phenotype should not be confused with the presence of emphysema, which may be present in any of the phenotypes and even in smokers without criteria for COPD.
The emphysema phenotype usually has fewer exacerbations than the chronic bronchitis phenotype, but it is possible that patients with emphysema are also exacerbators, especially those with severer forms of the disease.21 Severe emphysema is also associated with a poor prognosis as it is a predictor for a greater annual fall in FEV1.22
Definition of the Chronic Bronchitis PhenotypeChronic bronchitis was defined in the 1958 Ciba Symposium, and ratified by the WHO in 1961 and by the ATS one year later, as the presence of productive cough or expectoration for more than three months a year during more than two consecutive years.23 The chronic bronchitis phenotype identifies COPD patients in whom chronic bronchitis is the predominant symptom. Bronchial hypersecretion in COPD has been associated with greater airway inflammation and greater risk for respiratory infection,24 which can explain why patients with chronic bronchitis have a greater frequency of exacerbations than patients without chronic expectoration.25–27 A significant number of patients with chronic bronchitis and repeated exacerbations may be seen to have bronchiectasis when studied with thoracic high-resolution computed tomography (HRCT).28,29
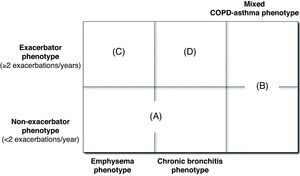
Phenotype CharacterizationThe mixed, emphysema and chronic bronchitis phenotypes are mutually exclusive and the diagnosis is based on the predominant clinical manifestations and the compliance of the diagnostic criteria. The exacerbator phenotype coexists with the three previous phenotypes, creating 4 phenotypic combinations with different management (Fig. 1), according to the diagnostic algorithm presented in Fig. 2.
- •
Type A: non-exacerbator COPD with emphysema or chronic bronchitis.
- •
Type B: mixed COPD with asthma, with or without frequent exacerbations.
- •
Type C: exacerbator COPD with emphysema.
- •
Type D: exacerbator COPD with chronic bronchitis.
There may be cases that are difficult to classify that share characteristics of more than one phenotype. In this instance, we should pay attention to the most important problem for the patient. First of all, if he/she presents with frequent exacerbations, we should aim the treatment toward prevention. Secondly, if there are signs of mixed phenotype, we should try to treat the inflammatory component. In patients with chronic bronchitis, it is possible to discover emphysematous lesions on chest CT, but the presence of cough with expectoration will continue to be the main symptom that classifies these patients as chronic bronchitis phenotype.
Can the Phenotype Change?Despite the fact that phenotypes are generally stable, they may change their expression either spontaneously or due to the effects of the treatment. For example, an exacerbator patient can stop suffering exacerbations or a mixed patient may have a negative bronchodilator test and lower eosinophilic inflammation thanks to treatment. In cases in which the changes are due to the treatment, it is recommended to continue with the same dosage.
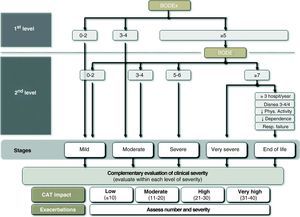
Severity Classification According to GesEPOCFollowing the principles of multidimensional assessment, GesEPOC proposes a severity classification with 5 different levels, whose main determinant for severity is the BODE index and its different quartiles.30 With the lack of information about the distance walked in the 6-min walk test, GesEPOC recommends using the BODEx index as an alternative only for levels I and II (mild or moderate COPD).31 All the patients with a BODEx of 5 or more points should do the exercise test in order to specify their level of severity. The health-care centers that do not provide this test should consider sending the patient to a secondary health-care center. GesEPOC proposes a fifth level of severity aimed at identifying patients with a high risk for death or those who are in the last stage of life and may benefit from a multidimensional evaluation by teams who are experts in palliative care. The criteria for recognizing level V are included in Table 2.
Criteria for Stage V Severity: COPD at the End of Life.
| BODE≥7 points and also at least one of the following: |
| ≥3 hospitalizations per year |
| Dyspnea 3 or 4 on the mMRC scale, despite optimal treatment |
| Sedentarism or low physical activity |
| High dependence for daily activities |
| Chronic respiratory insufficiency |
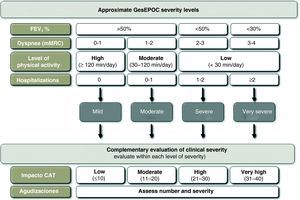
Multidimensional indices have a closer relationship with the prognosis of COPD than any individually considered variable. However, there still is no evidence to show that the treatment directed by these indices can achieve better clinical results than that oriented by classic symptoms and pulmonary function. In addition, the implementation of the BODE/BODEx indices in clinical practice requires familiarization with the use of risk scales. This approach may require a period of adaptation, and therefore GesEPOC suggests an alternative approach that may be indicative of the severity of the patient and is more intuitive based on the usual data recorded at the office visit. The latest GOLD 2011 guidelines, although with a slightly different approach, also recommends carrying out a multidimensional evaluation which, in addition to FEV1, contemplates exacerbation frequency, symptoms (dyspnea) and the score from the COPD Assessment Test (CAT).7
The variables that can be used for this approach to determine severity level are: airflow obstruction, measured by FEV1 (%); dyspnea, measured by the mMRC scale; level of physical activity; and hospitalizations in the previous two years. In accordance with the GOLD guidelines, the cut-point of FEV1 (%)=50% is considered the threshold for determining a patient as either severe or very severe.7 The mMRC dyspnea scale approximates the severity of patients.32 Several studies have demonstrated the prognostic value of dyspnea, which in some studies even surpasses FEV1 (%) in predictive value for mortality.33 The measurement of physical activity also has a very important prognostic value in COPD for exacerbations, loss of lung function, hospitalizations and finally mortality.34,35 In order to evaluate physical activity, the measurements that are self-reported by the patients are simple, available at all health-care levels and have offered very good results as predictors for severity outcomes (hospitalization and death).34 For measuring physical activity, we propose calculating the minutes of daily physical activity at an intensity of more than 3.0 METs36 or daily minutes of moderate physical activity. As for hospitalizations, it has been demonstrated that these are a very important risk factor for future mortality in patients with any level of airflow obstruction severity.37 Moreover, such circumstances are easy for patients to remember. Cut-points are shown in Fig. 3.
It should be remembered that not one parameter alone can classify COPD severity level. If the BODE index or BODEx is calculated, the physician who treats the patient should consider all the aspects enumerated and classify the patient into one of the GesEPOC severity levels according to an overall assessment of all the factors. In any event, the calculation of the BODE index or BODEx will determine the definitive severity classification and their use is recommended as a first option.
Adjusting the Treatment Intensity for Each COPD Severity LevelThe choice of treatment should be based on the clinical phenotype of the patient and the intensity is determined by the multidimensional level of severity, following the previous outline. Nevertheless, within one same level of severity, there are other parameters that can modulate the intensity of the treatment. Among these are the severity of the symptoms, the frequency and intensity of the exacerbations or the deterioration in health-rated quality of life using the CAT questionnaire (COPD Assessment Test). The CAT is a standardized questionnaire that is short and simple, which has recently been developed to be used in clinical practice.38,39 Currently, there are no CAT thresholds that can recommend a modification in the therapeutic dosage, although the 2011 GOLD Guidelines recommend using 10 units as a cut-point for severity in order to intensify the treatment. In order to specify the severity of the patients and the impact of the disease, the thresholds used in the development and validation of the questionnaire are recommended (www.catestonline.org) (Fig. 4).39
- •
Treatment of stable COPD is based on long-acting bronchodilators (LABD).
- •
Drugs that should be added to the LABD therapy depend on the patient phenotype.
- •
Treatment of the non-exacerbator phenotype, either emphysema or chronic bronchitis, is based on the use of combined LABD.
- •
Treatment of the mixed phenotype is based on the use of LABD combined with inhaled corticosteroids (IC).
- •
Treatment of the exacerbator phenotype with emphysema is based on LABD, to which IC and theophylline can be added according to the level of severity.
- •
When treating the exacerbator phenotype with chronic bronchitis, IC, phosphodiesterase IV inhibitors or mucolytic agents can be added to the LABD, depending on severity. In special cases, preventive antibiotics can be used.
- •
Special attention should be paid to comorbidities, and their control optimized.
The general objectives of COPD treatment are summarized into three: reduce the chronic symptoms of the disease, reduce the frequency and severity of the exacerbations and improve prognosis. Both short-term benefits (control of the disease) as well as mid- and long-term objectives (reduced risk for exacerbations, accelerated loss in lung function or death) should be reached.7,40
There is a series of general measures to keep in mind for all COPD patients that include quitting smoking, proper nutrition, regular physical activity, evaluation and treatment of comorbidities and vaccinations, which will not be dealt with in this publication, but which are described in greater detail in the publication of the complete guidelines.
Treatment of Type A COPD: Exacerbator Phenotype with Emphysema or Chronic BronchitisThe treatment of this phenotype includes one or two bronchodilators from different therapeutic groups. The patients who do not present exacerbations do not have an indication for either anti-inflammatory or mucolytic treatment.
Short-acting BronchodilatorsThe first step in the treatment of COPD is bronchodilation. Short-acting bronchodilators (anticholinergics [short-acting muscarinic antagonists, SAMA] such as ipratropium bromide and short-acting β2 agonists [SABA] such as salbutamol or terbutaline) are effective drugs that quickly control symptoms. These drugs, added to the basic therapy, are the first-choice treatment for symptoms on demand, whatever the severity of the disease.41
When the patient has frequent symptoms, requires frequent treatment with short-acting bronchodilators or suffers exercise limitations, regular treatment is necessary. In this case, a long-acting bronchodilator (LABD) should be prescribed.
Long-acting BronchodilatorsLABD can be long-acting β-agonists (LABA−salmeterol, formoterol and indacaterol) or long-acting muscarinic antagonists (LAMA−tiotropium bromide). They should be used as a first step in the treatment of all patients with permanent symptoms who regularly require treatment because they provide greater control over the symptoms and improve quality of life as well as pulmonary function and exercise tolerance, while also reducing exacerbations.42–46 There are differences between the different LABD: the action of some lasts for 12h (salmeterol and formoterol), while others last 24h (tiotropium and indacaterol). Formoterol and indacaterol begin to act quickly, and tiotropium and salmeterol initiate their bronchodilator action more slowly. The comparisons between LAMA and LABA in two systematic reviews47,48 did not show differences in the frequency of exacerbations between the two treatments. Differences were found, however, in the subanalysis of the patients with FEV1≤40%, where tiotropium was more effective in reducing exacerbations.47 More recently, a randomized clinical assay (RCA) about exacerbations showed that tiotropium was more effective than salmeterol for preventing exacerbations in patients with COPD and a history of at least one exacerbation in the previous year.49 These results correspond with comparisons between tiotropium and salmeterol, but there are no studies in the literature comparing tiotropium and indacaterol for preventing exacerbations.
Double Bronchodilator TherapyIn symptomatic patients or in those with evident exercise limitations, even after bronchodilator monotherapy, double bronchodilator therapy should be tried. The association of LABA and LAMA offers an added functional benefit with a reduced need for rescue medication, improved symptoms and quality of life compared with monotherapy.50,51 For this reason, in a second treatment step for patients with a severity level II or higher, another LABD can be associated that is pharmacologically different to what the patient was taking when at level I, be it either LAMA or LABA. In this manner, the bronchodilator effect is optimized.
TheophyllinesTheophyllines are weak bronchodilator drugs, but they present additional effects to the standard bronchodilators. These drugs have been reported to have a positive effect on diaphragm strength, better performance of respiratory muscles, reduction in air trapping, an improvement in mucociliary clearance and a reduction in exacerbations.52 In any event, their limited clinical efficacy and narrow therapeutic margin relegate theophyllines to the second line of importance, especially in severe patients with severity levels IV or V.53
Substitutive Treatment with alpha-1-antitrypsinSubstitutive treatment with purified alpha-1-antitrypsin (AAT) is recommended by the main scientific societies (American Thoracic Society, European Respiratory Society and the Spanish Society of Pulmonology and Thoracic Surgery) in patients with pulmonary emphysema with severe AAT deficiency and with PiZZ homozygous phenotype or rare deficiency variations. The inclusion and exclusion criteria are well defined in the specific guidelines.54,55
All COPD patients, especially in emphysema phenotypes, should have at least one measurement of alpha-1-antitrypsin serum concentrations in order to rule out that he/she could have a deficiency of this enzyme.
Treatment of Type B COPD: The Mixed COPD-Asthma PhenotypeIt is possible that a patient with mixed COPD-asthma phenotype may be cataloged as such or instead as an asthmatic smoker with obstruction that is not completely reversible.56 Their main characteristic is that they present a greater degree of bronchial eosinophilic inflammation that would be responsible for the greater clinical and spirometric response to inhaled corticosteroids (IC).57,58 The use of IC associated with LABD is also justified as a first option at severity level I or II with the aim to improve lung function, respiratory symptoms and to reduce exacerbations, if there were any.11,59
In cases with greater severity (severity levels III and IV), it may be necessary to use triple treatment: IC/LABA plus LAMA. This triple combination has been demonstrated to be effective in patients with COPD who presented much airflow obstruction reversibility.60 In addition, tiotropium has been shown to be effective in asthma patients.61
Also, in more severe cases (severity level IV) theophylline may be added to the treatment, as can roflumilast if there is chronic cough with expectoration. There are no specific studies of the efficacy and safety of these drugs in severe COPD with mixed phenotype, but we know the effectiveness of both drugs in asthma.
Treatment of Type C COPD: Exacerbator Phenotype with EmphysemaPatients with emphysema can also be exacerbators and require a treatment directed at reducing the number of exacerbations as well as improving other disease parameters. LABD in the first step of treatment (severity level I–II), either alone or combined, are effective at reducing exacerbations. But, in some patients these are insufficient and will require an intensification of the pharmacological treatment.
Inhaled CorticosteroidsDifferent clinical practice guidelines5–7 recognize the usefulness of using IC in patients who present frequent exacerbations in spite of optimal bronchodilator treatment, and their use associated with LABD produces a significant decrease in the number of exacerbations and an improved quality of life, even though they have not been shown to have a beneficial effect on mortality.62–64 Although the prevention of exacerbations has been evaluated in most studies for severe or very severe exacerbator patients (degree of obstruction III and IV, FEV1<50%), there are some studies in patients with less functional severity (FEV1<60%) where the results also support the use of these drugs. Therefore, it seems that the main determinant of the benefit is the presence of repeated exacerbations, meaning the exacerbator phenotype, and not the degree of airflow obstruction.62 Thus, they may be tried in patients at severity level II who persist with exacerbations despite treatment with one or two long-acting bronchodilators. In COPD, IC should always be used in association with LABD.In patients at severity level III who do not present a level of control of the symptoms or exacerbations with two drugs (either two LABD or one LABD plus an IC), triple therapy (LAMA+LABA+CI) can be used. The few studies that have been published with triple therapy indicate a greater effect on lung function and a reduction in exacerbations and hospitalizations in severe patients.60,65
Treatment of Type D COPD: The Exacerbator Phenotype with Chronic BronchitisThe presence of cough and chronic expectoration is a known factor that predisposes patients to exacerbations in COPD.25 The first step in the treatment of severity level I entails LABD due to their capability to reduce the number of exacerbations. In severity level II, double therapy is recommended with two LABD or with one LABD plus an anti-inflammatory drug, either IC or roflumilast.
Inhibitors of the Phosphodiesterase 4: RoflumilastRoflumilast is an oral anti-inflammatory drug that acts by selectively inhibiting phosphodiesterase 4 (IPD4). It has been shown to prevent exacerbations in patients with severe COPD who present cough and chronic expectoration and also suffer frequent exacerbations66,67; hence, it is a drug indicated for the exacerbator phenotype with chronic bronchitis. This effect is maintained when roflumilast is added to the maintenance treatment with an LABD, either LABA or LAMA. In addition, it reaches a significant increase in the FEV1 valley of between 50 and 70mL above that reached with salmeterol or tiotropium.68,69
Both roflumilast and CI are anti-inflammatory drugs, although their modes of action are different. The results obtained in clinical assays with the concomitant administration of IC and roflumilast indicate that this association is safe and that roflumilast maintains its clinical efficacy.67 It may be useful when considering associating both anti-inflammatory actions in patients at a high risk for exacerbations, although always associated with LABD. The use of roflumilast is not recommended with theophyllines.
Mucolytic AgentsTwo systematic reviews demonstrated a reduction in exacerbations with mucolytic treatment compared with placebo in COPD patients.70,71 These results should be interpreted with caution as the studies included had a limited sample size and were heterogeneous. However, the same results were confirmed in a larger clinical assay in which the use of long-term carbocisteine, compared with placebo, reduced the number of exacerbations, delayed the progressive worsening of the symptoms and improved the quality of life of the COPD patients.72 The effects of long-term N-acetylcysteine (NAC) in COPD patients have been evaluated in a clinical assay, in which there was a demonstrated decrease in the number of exacerbations in patients who were not treated with concomitant IC.73 Nevertheless, the evidence is insufficient to be able to generate a recommendation about the effects of NAC in COPD patients who are not treated with IC.
Carbocisteine can be used as a second line of treatment in patients with severity levels III and IV who have frequent exacerbations despite optimal bronchodilator treatment.
Antibiotics in Stable COPDThe use of antibiotics in stable COPD has been done empirically since the 1960s in what was known as antibiotic prophylaxis. A systematic review of the assays done up until the 1980s concluded that there was a small but significant beneficial effect in the reduction of exacerbations.74 These studies included poorly defined populations, frequently patients with chronic bronchitis and no diagnostic confirmation of COPD.
In the last decade, strictly designed clinical assays have been carried out to determine the efficacy of antibiotics administered in stable phase for the prevention of exacerbations. We can divide these studies into two groups: (1) those who use macrolides with the intention of also taking advantage of their anti-inflammatory action; (2) those who use quinolones in order to achieve maximal bacterial eradication.
Macrolides, administered for prolonged periods and at low doses, due to their anti-inflammatory and immunomodulatory activity,75 have been shown to significantly reduce the number of exacerbations in stable patients with severe COPD.76–78 However, the populations studied and dosages were different, thus it is difficult to make a recommendation. It should be mentioned that the study by Albert et al.78 confirmed an increase in the bacterial resistances to macrolides and a slight increase in auditory problems in patients treated with azithromycin.
The use of quinolones during periods of stability (treatment of chronic bronchial infection) has been shown to eradicate the bacteria present in the sputum of most patients with severe COPD, chronic bronchial infection and frequent exacerbations.79 The administration of moxifloxacin 5 days a month every two months during one year reduced by 45% the number of exacerbations in those patients who presented with purulent sputum, which are patients with a greater probability of having chronic bacterial bronchial infection. In this study, no evidence of a significant increase in resistances was found.80
These treatments will be reserved for very select cases of patients with a level of severity of IV and frequent exacerbations that required multiple antibiotic treatments or hospitalizations the previous year in spite of correct treatment of the COPD. In addition, its use should be restricted to reference centers with clinical, auditory and hepatic biochemistry follow-up and microbiology with the identification of microorganisms in the sputum and a study of the sensitivity to antibiotics.
Patients who are candidates for chronic or cycled treatment with antibiotics are patients with a high probability of being carriers of bronchiectasis,28,29 and bronchiectasis treatment guidelines can be applied in order to control the chronic bronchial infection.81
A summary of the pharmacological treatment by phenotypes and level of severity are shown in Table 3.
Pharmacological Treatment of COPD According to Phenotypes and Levels of Severity (I-IV).
| Severity Stage | ||||
| Phenotype | I | II | III | IV |
| ANon-exacerbator with emphysema or CB | LAMA or LABASABA or SAMAa | LAMA or LABALAMA+LABA | LAMA+LABA | LAMA+LABA+theophylline |
| BMixed COPD-asthma | LABA+IC | LABA+IC | LAMA+LABA+IC | LAMA+LABA+IC (consider evaluating theophylline or PDE4 inhibitor if there is expectoration) |
| CExacerbator with emphysema | LAMA or LABA | (LABA or LAMA)+ICLAMA+LABALAMA or LABA | LAMA+LABA+IC | LAMA+LABA+IC (consider adding theophylline) |
| DExacerbator with CB | LAMA or LABA | (LAMA or LABA)+(IC or IPE4)LAMA+LABALAMA or LABA | LAMA+LABA+(IC or IPE4)(LAMA or LABA)+IC+IPE4(consider adding carbocisteine) | LAMA+LABA+(IC or IPE4)LAMA+LABA+IC+IPE4(consider adding carbocisteine) (consider adding theophylline)(consider adding antibiotics) |
Abbreviations. CB: chronic bronchitis; SABA: short-acting beta-2 agonist; SAMA: short-acting muscarinic antagonists; IC: inhaled corticosteroid; LAMA: long-acting muscarinic antagonists; LABA: long-acting beta-2 agonist; PDE4: phosphodiesterase 4.
The need to increase treatment as the disease progresses has been well established. There is, however, very limited evidence about the possible reduction or withdrawal of treatment in COPD patients who either improve or become clinically stable. The following recommendations are based on this limited evidence:
- (a)
Bronchodilator treatment only has an effect when it is being administered. Thus, it is very probable that the withdrawal of a bronchodilator or its substitution for another bronchodilator that is either not as strong or does not last as long would cause lung function and/or symptoms to worsen.82
- (b)
In mixed phenotype patients, an attempt may be made at reducing the IC dosage until the minimal effective dose is determined, as is done in asthma. It is not recommended to prescribe these patients LABD treatment without IC.
- (c)
In patients with the exacerbator phenotype, it is not possible to specify a way to reduce the treatment in cases of stability. Before even considering a reduction in treatment, the patient should have had at least one exacerbation-free year. The process should be done according to the judgment of the clinician, starting with the withdrawal of those medications that are probably less active or present a greater probability of side effects over the short or long term.
- (d)
In mild to moderate patients (severity level I–II) without mixed phenotype and who continue treatment with IC at high doses, the need to continue with these drugs should be reevaluated. There are studies that suggest that the abrupt withdrawal of IC can trigger an exacerbation,83 although a recent systematic review concludes that there is not sufficient evidence to relate the withdrawal of IC with exacerbations.84 In any event, the indication should be individualized, and never in patients who continue to have a positive bronchodilator test or eosinophilia in sputum despite treatment with IC.85,86 It should only be done in stable patients who have not had exacerbations for at least one year, not during the winter (when there is a higher incidence of exacerbations),86 with a progressive reduction of the dose that is closely controlled both clinically and by spirometry.
The treatment of COPD in stable phase has experienced important changes in recent years derived from the introduction of new drugs and the publication of new clinical assays and meta-analyses, some of patients with specific characteristics. These advances require us to reconsider the approach toward treatment following a strategy based on clinical phenotypes that characterize and group COPD patients who present a certain response to treatment. A new approach would also entail a series of recommendations that still are not supported by a high grade of evidence but, as stated in the new GOLD guidelines, “lack of evidence of the effectiveness of a particular treatment is not the same as evidence that it is not effective”.7 Based on the best available evidence, GesEPOC was developed. The complete document discusses new lines of research that will help improve this proposal in the future.
The GesEPOC OrganizationCoordinator: Marc Miravitlles, Sociedad Española de Neumología y Cirugía Torácica (SEPAR). Members of the workgroup: Myriam Calle y Juan José Soler-Cataluña (SEPAR); Joan B. Soriano (SEPAR-epidemiology); Julio Ancochea, scientific coordinator of the COPD Strategy of the SNS; Pere Almagro, Sociedad Española de Medicina Interna (SEMI); Daniel López (SEPAR-Physiotherapy); Esther Marco, Sociedad Española de Rehabilitación y Medicina Física and Sociedad de Rehabilitación Cardio-Respiratoria (SERMEF/SORECAR); Juan Antonio Riesco, Comité Nacional de Prevención del Tabaquismo (CNPT); José Antonio Quintano, Sociedad Española de Médicos de Atención Primaria (SEMERGEN); Juan Antonio Trigueros, Sociedad Española de Médicos Generales y de Familia (SEMG); Jesús Molina, Sociedad Española de Medicina de Familia y Comunitaria (semFYC) and Sociedad de Respiratorio en Atención Primaria (GRAP); Mercè Marzo (semFYC-Metodology); Pascual Piñera and Adolfo Simón, Sociedad Española de Medicina de Urgencias y Emergencias (SEMES); Antonia Cachinero (SEPAR-Nursing); María Dolors Navarro, Foro Español de Pacientes (FEP); Montse Llamas (UOC – WestWing – communications).
GESEPOC has also been supported by strategical partners: Almirall, AstraZeneca, Boehringer Ingelheim-Pfizer, Faes Farma, Grupo Ferrer, GlaxoSmithKline, Novartis and Nycomed-Merck Sharp & Dhome. Collaborators included: Chiesi, Esteve Teijin and Grupo Uriach Pharma.
Conflicts of InterestMarc Miravitlles has been paid to give conferences at educational events organized by Boehringer Ingelheim, Pfizer, AstraZeneca, Bayer Schering, Novartis, Talecris, Takeda-Nycomed, Merck, Sharp & Dohme and Novartis, and for scientific consultation with Boehringer Ingelheim, Pfizer, GSK, AstraZeneca, Bayer Schering, Novartis, Almirall, Merck, Sharp & Dohme and Takeda-Nycomed. Juan José Soler has been paid to give conferences at educational events and/or for scientific assessment and/or research by Boehringer Ingelheim, Pfizer, AstraZeneca, Bayer Schering, Novartis, Takeda-Nycomed, Merck, Sharp & Dohme, Almirall, Grupo Ferrer, GSK and Vifor Pharma. Myriam Calle has been paid to give conferences at educational events and/or for scientific consultation and/or research by Carburos Médica, AstraZeneca, Merck, Sharp & Dohme and Almirall. Jesús Molina has no conflicts of interest to declare. Pere Almagro has been paid to give conferences at educational events and/or for scientific consulting and/or research by Boehringer Ingelheim, Pfizer, Takeda-Nycomed, Merck, Sharp & Dohme, Almirall, GSK, Chiesi and Esteve. José Antonio Quintano and Juan Antonio Riesco have no conflicts of interest to declare. Juan Antonio Trigueros has been paid to give conferences at educational events and/or for scientific consulting and/or research by Boehringer Ingelheim, Pfizer and Bayer Schering. Pascual Piñera and Adolfo Simón have no conflicts of interest to declare. José Luis López-Campos has been paid to give conferences at educational events and/or for scientific consulting and/or research by Boehringer Ingelheim, Pfizer, AstraZeneca, Novartis, Takeda-Nycomed, Merck, Sharp & Dohme, Almirall, GSK, Esteve and Faes Farma. Joan B. Soriano has been paid to give conferences at educational events and/or for scientific consulting and/or research by Almirall. Julio Ancochea has been paid to give conferences at educational events and/or for scientific consulting and/or research by Boehringer Ingelheim, Novartis, Takeda-Nycomed, Almirall, GSK, Intermunne, Faes Farma, Chiesi and Actelion.
The authors would like to thank the following experts for their collaboration in reviewing the manuscript: Pilar de Lucas (Madrid), José Luis Izquierdo Alonso (Guadalajara), Germán Peces-Barba (Madrid), Francisco Casas Maldonado (Granada), Luis Muñoz (Córdoba), Borja García-Cossio (Palma), Cristóbal Esteban (Galdakano), Adolfo Baloira (Pontevedra), Luis Pérez de Llano (Lugo), Ramón Agüero (Santander), Teodoro Montemayor (Seville), José Luis Viejo Bañuelos (Burgos), Carlos Álvarez (Madrid), Francisco García Río (Madrid), Luis Puente Maestu (Madrid), Alfredo de Diego (Valencia), José Miguel Rodríguez González-Moro (Madrid).
Please cite this article as: Miravitlles M, et al. Guía Española de la EPOC (GesEPOC). Tratamiento farmacológico de la EPOC estable. Arch Bronconeumol. 2012;48:247–57.
The original Spanish article was published simultaneously in Atención Primaria. Aten Primaria. 2012. http://dx.doi.org/10.1016/j.aprim.2012.04.005.
The following scientific societies and institutions have been involved in this consensus document: SEPAR, SEMI, SERMEF/SORECAR, CNPT, SEMERGEN, SEMG, semFYC, GRAP, SEMES, SEPAR-ENFERMERÍA, FEP and UOC.