Recently, a number of reports have been published on silicosis in workers exposed to artificial quartz conglomerates containing high levels of crystalline silica particles (70%–90%) used in the construction of kitchen and bathroom surfaces. Three cases of silicosis in workers exposed to artificial quartz conglomerates are reported. The diagnosis was derived from both the International Labour Office and the International Classification of HRCT for Occupational and Environmental Respiratory Diseases (ICOERD) classifications and cytological analysis of bronchoalveolar lavage fluid. In 2 cases, levels of respirable silica greatly in excess of recommended standards were measured in the workplace, and cytological analysis of bronchoalveolar lavage fluid highlighted a prevalence of lymphocytes, meeting criteria for the diagnosis of accelerated silicosis.
The prevention of pneumoconiosis caused by the use of innovative materials such as artificial conglomerates with high crystalline silica content must be addressed.
En la bibliografía, hay una serie de estudios recientes sobre silicosis en trabajadores expuestos a conglomerados artificiales de cuarzo con un alto porcentaje de partículas de sílice cristalina (70–90%) empleado para elaborar superficies de cocinas y baños. Se analizan tres casos de silicosis en trabajadores expuestos a conglomerados artificiales de cuarzo. El diagnóstico se realizó conforme a las clasificaciones de la Organización Internacional del Trabajo y la ICOERD (clasificación internacional de TAC de alta resolución para enfermedades respiratorias ocupacionales y ambientales) y mediante análisis citológico del líquido del lavado broncoalveolar. En dos casos, se midieron en el lugar de trabajo niveles de sílice respirable que superaban en gran medida los umbrales recomendados y el análisis citológico del líquido del lavado broncoalveolar mostró la prevalencia de linfocitos indicativos de diagnóstico de silicosis acelerada.
Es preciso reestudiar esta neumoconiosis, sobre todo para evitar el uso de materiales innovadores como los conglomerados artificiales con alto contenido en sílice cristalina.
Silicosis occurs after inhalation of crystalline silica found in various minerals, such as quartz, sandstone and granite. Traditionally, a number of working populations were considered at risk. The disease is endemic worldwide, but incidence in Western countries and particularly in Europe has decreased over time. More recently, a number of reports have been published on silicosis in workers exposed to artificial quartz conglomerates containing high levels of crystalline silica particles (70%–90%), used in the construction of kitchen and bathroom surfaces.1–5 However, these studies did not report the analysis of environmental levels or specimens from personnel in the assessment of subjects’ exposure to silica. Diagnosis of silicosis was generally based on chest X-rays performed during routine medical examinations ordered by the employer. In a recent report from the province of Cádiz, Pérez-Alonso et al. used HRCT in the diagnosis of 46 cases of silicosis in subjects exposed to artificial quartz conglomerates. Of these, 91% had simple chronic silicosis.5 García-Vadillo et al. confirmed a histological diagnosis of silicosis in 4 workers by pulmonary biopsy.1 In these studies, simple silicosis appears to be the most common disease type.2,5
In Europe, the prevention of silicosis caused by the use of innovative materials must be addressed, particularly at the manufacturing stage, where exposure monitoring is uncommon. The risk derived from exposure to these new products appears to be higher than the risk from exposure to natural stone, and there may be significant differences between the two in terms of latent disease and clinically patent disease.
The use of chest X-ray in the diagnosis of dust-induced lung disease has been shown to be less sensitive and specific than high-resolution computed tomography (HRCT).6 On the other hand, cell patterns in bronchoalveolar lavage fluid (BALF), when used in conjunction with comprehensive clinical information and appropriate thoracic imaging such as HRCT, frequently provide useful information for the diagnostic evaluation of patients with interstitial lung diseases in general.7
We report three cases of silicosis in workers exposed to artificial quartz conglomerates. Diagnosis was obtained following both the International Labour Office (ILO)8 and the International Classification of HRCT for Occupational and Environmental Respiratory Diseases (ICOERD)9 guidelines and cytological analysis of BALF.
Case 1A 44-year-old man, non-smoker, who since 1988 has worked in a small ornamental stone company. His duties consisted of cutting, polishing and home installation of bathroom and kitchen surfaces. A water-jet cutting system was introduced in 1998. However, neither the water-jet cutting system nor personal protection was used during finishing and home installation. Since 2005, the subject has intensively used artificial quartz conglomerates for constructing kitchen and bathroom surfaces. Data combining indoor environmental measurements and monitoring of personnel (in accordance with UNI EN 689/1997) obtained from this factory in 2012 showed an average crystalline silica concentration in the finishing area of >0.5mg/m2. The critical level still ranges far above the legal threshold, according to both the TLV ACGIH (0.025mg/m2) and the European Scientific Committee Exposure Limit (SCOEL) (0.05mg/m2). After the environmental monitoring campaign, preventive measures were introduced according to NEPSI Good Practices (see “Discussion” section).10
The patient was asymptomatic and lung function test results were normal. Chest X-ray performed in 2012 during routine medical examinations ordered by the employer revealed diffuse nodules of less than 1cm in size. ILO classification showed nodular q-q opacities with 2/2 profusion in both lungs and lymph nodes with eggshell calcification. Lung HRCT according to ICOERD classification showed prevalent well-defined rounded grade 2 opacities, predominantly size q, profusion grade 8. ANA (antinuclear antibody), anti-ENA (extractable nuclear antigens) antibody and ACE (angiotensin-converting enzyme) values were normal. QuantiFERON testing for Mycobacterium tuberculosis was negative. Mycobacteria detection with direct microscopy, culture and polymerase chain reaction (PCR) and bacterial and mycological culture of BALF were all negative. BALF cytology showed a significant predominance of pigmented alveolar macrophages (Perls stain positive), associated with a low number of birefractive bodies (compatible with silica, but not indicative of silica exposure). Cell count showed lymphocytosis (35%) with a normal CD4+/CD8+ratio.
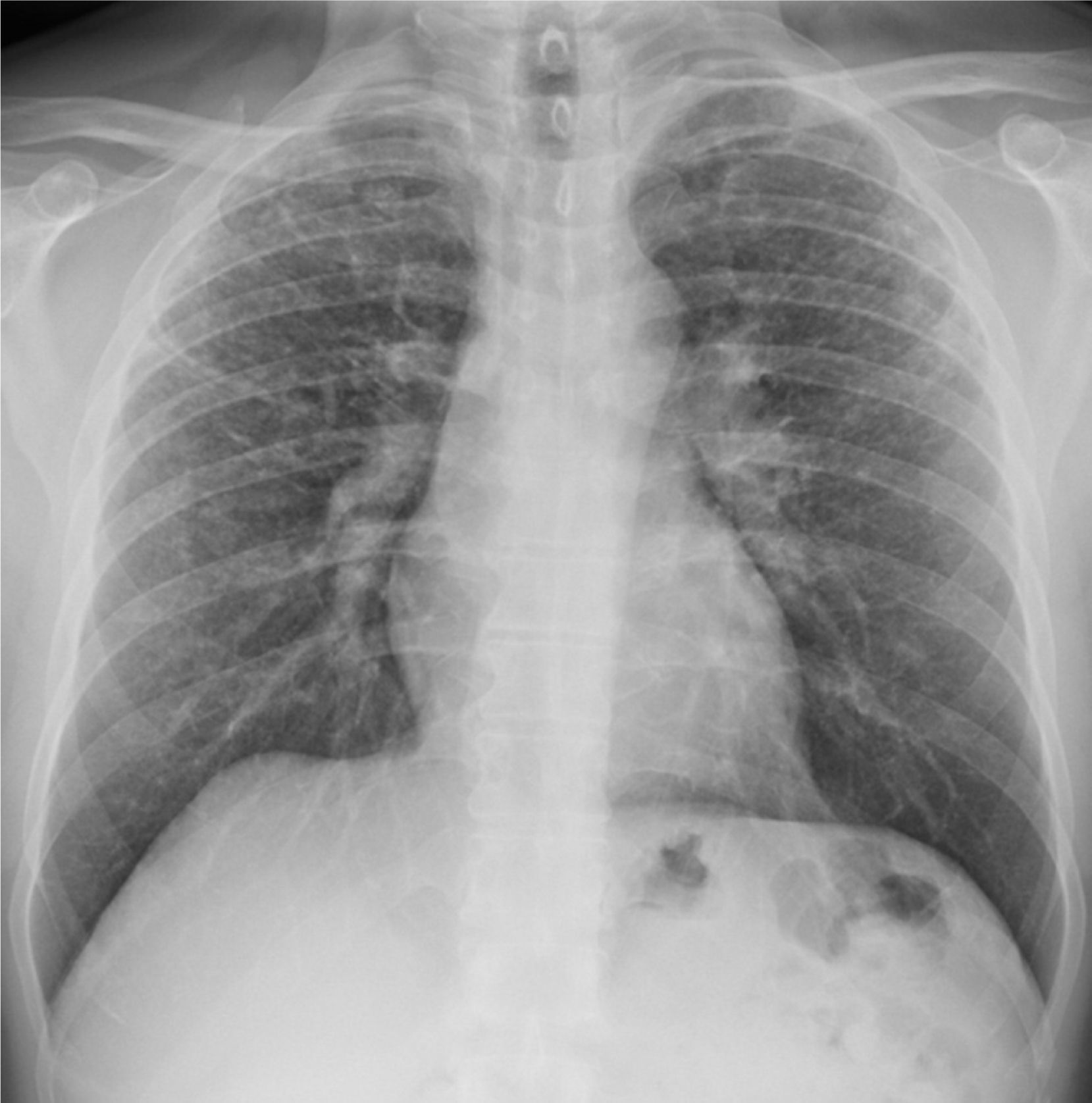
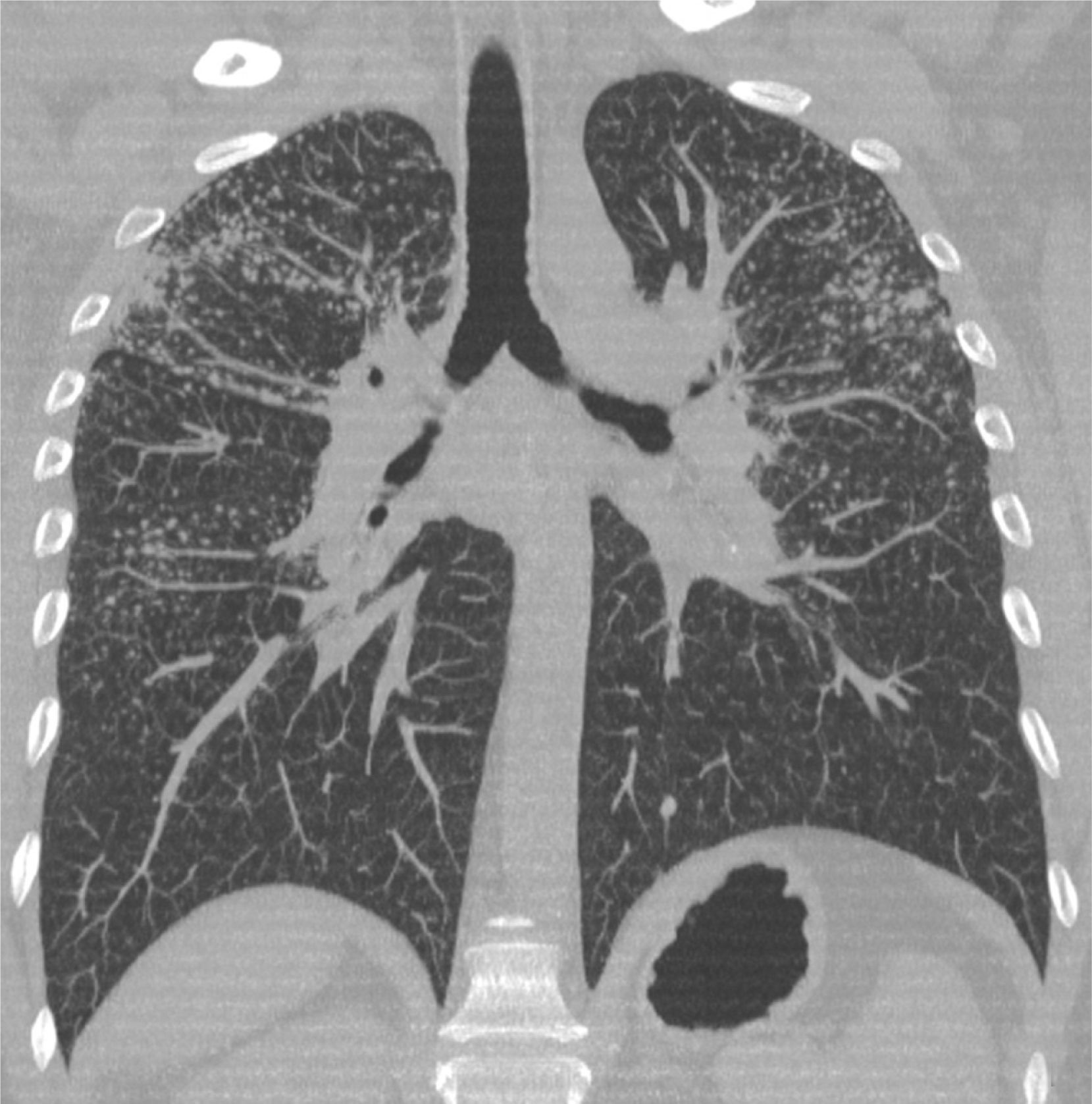
Case 2A 50-year-old man, non-smoker, who since 1981 has worked in the same company as Case 1, performing similar tasks. Since 2000, he has intensively used artificial quartz conglomerates for constructing kitchen and bathroom surfaces. The patient was asymptomatic and lung function test results were normal. Chest X-ray was performed twice, in 2011 (during routine medical examinations ordered by the company) and in 2013 (Fig. 1). In 2011, ILO classification showed nodular q-p opacities with 2/2 profusion in both lungs, mainly in the upper and middle lung zones. In 2011, lung HRCT according to ICOERD classification showed prevalent well-defined rounded grade 2 opacities, predominantly size q, profusion grade10 (Fig. 2). The patient was assigned to different tasks in order to prevent further exposure, but in 2013 the radiographic abnormalities according to ILO classification had increased (q/r opacities with profusion 2/3 in both lungs and large type A opacities). ANA and anti-ENA levels were normal. QuantiFERON testing for Mycobacterium tuberculosis was negative. Mycobacteria detection with direct microscopy, culture and PCR, and bacterial and mycological culture of BALF were all negative. Cell count showed lymphocytosis (41%) and a decreased CD4+/CD8+ratio.
Case 3A 55-year-old man, non-smoker, who since 1986 has worked in a small ornamental stone company. His main duty consisted of cutting stone with a water-jet system, occasional polishing, and installing artificial quartz conglomerate bathroom and kitchen surfaces. No protection was used during polishing and home installation. The patient was asymptomatic and lung function test results were normal. Chest X-ray according to ILO classification showed nodular p-p opacities with 1/0 profusion in both lungs, mainly in the upper lung zones. Lung HRCT according to ICOERD classification showed prevalent well-defined rounded grade 1 opacities, predominantly size p, profusion grade 4. ANA, anti-ENA and ACE levels were normal. QuantiFERON testing for Mycobacterium tuberculosis was negative. Mycobacteria detection with direct microscopy, culture and PCR, and bacterial and mycological culture of BALF were all negative. Cell count showed prevalent alveolar macrophages (88%).
DiscussionIn 2010, two Pubmed search string determinants (one more specific, the other more sensitive) were proposed for the retrieval of information on the possible association between occupational risk factors and some diseases.11 Using artificial conglomerates and silicosis, artificial conglomerate and silicosis, artificial stone and silicosis, quartz conglomerates and silicosis, quartz conglomerate and silicosis, 7 papers were found with the specific string (5 highly pertinent1–5) and 7 with the sensitive string (the same as those retrieved by the specific string). Articles primarily on Spanish workers in the stone cutting, shaping, and finishing industry were found. None of the cases reported in the literature were characterized by both HRCT classifications and BALF cell analysis.
An unusually high incidence of advanced life-threatening silicosis in workers exposed to artificial quartz conglomerates was reported in Israel.4 The first case in this epidemic of end-stage silicosis leading to lung transplantation occurred approximately 10 years after the commercial introduction of artificial quartz conglomerates, a short time given the severity of the disease. Authors used industrial hygiene principles to suggest that levels of respirable silica in excess of recommended standards may have been generated by the practice of dry stone cutting, as described by the patients. However, a limitation of this report, as pointed out by the authors, was the lack of dust exposure measurements.
Pérez-Alonso et al. reported that in a company in Cádiz, the level of free silica exceeded acceptable limits in 3 sampling points; however, the cited sources are not readily available.5 A study carried out in the Basque Country, also by Pérez-Alonso et al.,12 detected high levels of silica exposure in 20% of workplaces, mainly in artificial quartz conglomerate polishing tasks, where crystalline silica concentration in some cases was >0.4mg/m2.
In Cases 1 and 2, exposure to silica 20 times higher than the ACGIH TLV was demonstrated.10 Both patients presented a clinical picture of severe silicosis disproportional to exposure. On this basis, these cases were diagnosed as accelerated silicosis and prognosis was unfavourable.6 According to the ATS,7 BALF in chronic simple silicosis is characterized by a prevalence of alveolar macrophages. An increased number of lymphocytes and neutrophils seems to characterize the inflammatory process associated with progression to silicosis. In subjects affected by accelerated silicosis, prevalent lymphocytes are observed, as in Cases 1 and 2. This result might open new perspectives, both for determining prognosis and on the merits of follow-up programmes at least in serious cases occurring within a few years of exposure.
Data from the literature prove that dust from artificial quartz conglomerates is highly pathogenic.4,5 The extent of the damage cannot apparently be explained merely by the exposure level, and the role of the morphologic or chemical characteristics of the inhaled dust (shape, size, surface, chemical interaction) should also be taken into consideration. Recent advances point to the variable pathogenic potential of different varieties of silica and the direct role played by the silica particle surface in triggering adverse biologic reactions, such as ROS and RNS generation.13 These reactions can be modulated by the presence of transition metal ions.10 Studies on the biological mechanisms of silicosis are needed, in particular as regards the dust generated by cutting artificial conglomerates that contain not only silica but also resins.
Reducing exposure is a difficult challenge, but even so, preventive measures such as water-jet cutting systems and respiratory protection must be introduced in workplaces in accordance with European policies. On 25 April 2006, NEPSI (the European Network for Silica formed by the Employee and Employer European sectoral associations) signed the Social Dialogue titled “Agreement on workers’ health protection through the good handling and use of crystalline silica and products containing it”. The core text of this agreement contains the NEPSI Good Practice Guide which summarizes the principles of risk prevention in the use of products containing silica.14
Conflicts of InterestNo conflict of interests.
We thank Marzia Tarchi (Unit of Prevention in the Workplace, AUSL 11 Empoli, Italy) and Giuseppina Scancarello (Laboratory of Public Health, AUSL 7 Siena, Italy) for their kind collaboration.
Please cite this article as: Paolucci V, Romeo R, Sisinni AG, Bartoli D, Mazzei MA, Sartorelli P. Silicosis en trabajadores expuestos a conglomerados artificiales de cuarzo: ¿es distinta a la silicosis crónica simple? Arch Bronconeumol. 2015;51:e57–e60.