Sex and gender influence many aspects of chronic obstructive pulmonary disease (COPD). Limited data are available on this topic in alpha-1 antitrypsin deficiency (AATD). We therefore aimed to investigate sex issues in the EARCO registry, a prospective, international, observational cohort study.
MethodsBaseline data from PiZZ individuals, enrolled in the registry with complete data on sex and smoking history were analysed by group comparisons and binary logistic regression analyses.
Results1283 patients with AATD, 49.3% women were analysed. Females reported less tobacco consumption (16.8±12.2 vs. 19.6±14.5 PY, p=0.006), occupational exposures towards gases, dusts or asbestos (p<0.005 each) and consumed less alcohol (5.5±7.6 vs. 8.4±10.3u/week, p<0.001). Females reported COPD (41% vs. 57%, p<0.001) and liver disease (11% vs. 20%, p<0.001) less often. However, they had a higher prevalence of bronchiectasis (24% vs. 13%, p<0.001). Despite better lung function (FEV1%pred. 73.6±29.9 vs. 62.7±29.5, p<0.001) females reported a similar symptom burden (CAT 13.4±9.5 vs. 12.5±8.9, p=ns) and exacerbation frequency (at least one in the previous year 30% vs. 26%, p=ns) compared to males. In multivariate analyses, female sex was an independent risk factor for exacerbations in the previous year OR 1.6 p=0.001 in addition to smoking history, COPD, asthma and bronchiectasis and was also identified as risk factors for symptom burden (CAT≥10) OR 1.4 p=0.014 besides age, BMI, COPD and smoking history.
ConclusionMen had higher rates of COPD and liver disease, women were more likely to have bronchiectasis. Women's higher symptom burden and exacerbation frequency suggest they may need tailored treatment approaches.
Gender plays a role in many aspects of chronic obstructive pulmonary disease (COPD).1,2 Sex and gender in COPD reflect a complex interplay between genuine biological differences and sociocultural influences.2 In addition to possible biological aspects such as airway diameter or the complex effects of sex hormones on respiratory disease,3 socio-cultural factors also play an important role. Alpha-1-antitrypsin deficiency (AATD) is a genetic condition that predisposes to the early development of COPD4 and liver disease.5 Many of the issues related to sex and gender in COPD are plausibly important in AATD. For example, occupational exposures and certain risk behaviours, such as tobacco and alcohol consumption, for which gender differences still exist, have a significant impact on the development of AATD, lung and liver disease.6,7 The pattern of comorbidities differs significantly between men and women, but at the same time comorbidities play an important role in COPD and influence many of the symptoms that make up the individual burden of our patients.8 They also have a significant impact on the prognosis of the disease, and regardless of their prevalence, certain comorbidities are associated with different prognoses in men and women.9,10 To address the complexity of the relationship between gender and a genetic predisposition such as AATD, it is also important to recognise that men and women perceive disease differently, express symptoms differently and use health services in a different way.11 Due to the small number of cases in different countries and centres, it is especially difficult to study gender-specific aspects of rare diseases, and it is not surprising that there are no meaningful data on this topic in AATD.12 The prospective international registry of the European Alpha-1 Research Collaboration (EARCO) for patients with AATD offers the opportunity for the first time to investigate such questions in a large international, well-characterised cohort. The aim of the current study was to investigate sex-specific aspects of AATD in relation to lung and liver disease, symptom burden and exacerbations in the registry.
MethodsStudy populationBaseline data of patients from the prospective international registry for patients with AATD, EARCO were analysed. EARCO is a clinical research collaboration of the European Respiratory Society (ERS) with the aim of answering fundamental questions about the epidemiology, genetics, pathophysiology, clinical treatment and prognosis of AATD-associated lung diseases.13 The registry was originally designed as a European observational study, but has now grown beyond European borders to become a global registry.14 Individuals diagnosed with severe AATD, regardless of clinical features or severity, are eligible to participate in this observational study. The inclusion criteria are: (1) individuals diagnosed with AATD; (2) deficiency defined as AAT serum levels<11μM (50mg/dL) and/or proteinase inhibitor genotypes PI*ZZ, PI*SZ and heterozygous or homozygous combinations of other rare functionally deficient variants. Data on biological sex is completed by the enrolling healthcare professional; self-identified gender and differences from sex assigned at birth are not yet routinely collected. In the present analysis, only PI*ZZ individuals confirmed either by phenotyping or genotyping with complete information on gender and smoking status were included. The study protocol received central ethical approval from the Research Ethics Committee of the Vall d’Hebron University Hospital, Barcelona, Spain (PR(AG)480/2018) and was subsequently approved by all participating centres. Written informed consent was obtained from all participants. The EARCO registry protocol has been registered at www.clinicaltrials.gov (ID: NCT04180319).
AssessmentsDuring the annual visits of the EARCO study, a detailed recording of all AATD-specific characteristics, demographics, comorbidities, medication, generic and disease-specific quality of life, and psychological disorders was performed.12 Pulmonary function was assessed according to current guidelines.15,16 In addition to forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and its ratio FEV1/FVC, and total lung capacity (TLC) and residual volume (RV) were measured. For diffusion capacity, the transfer factor DLCO was used. In the current study, post-bronchodilator values were preferably used; in cases where these were not available, the values routinely recorded in the centres were used. The 6-minute walk test, which also complied with international recommendations, was carried out to assess exercise capacity.17 Standardised questionnaires on quality of life and symptoms, which were validated in several languages, were administered; the current analysis takes into account data from the disease-specific COPD Assessment Test (CAT),18 of the Modified Medical Research Council (mMRC)19 dyspnoea scale and the EuroQoL 5-dimension (EQ-5D) generic quality-of-life questionnaire.20
Statistical analysesMean values, standard deviations (SD), absolute numbers and percentages (%) were used to describe the data. For descriptive analyses group comparisons were made using Student's t-test or Mann–Whitney-U-test or Chi-squared test, as appropriate. Binary logistic regression analyses were performed to examine independent associations of symptoms and exacerbations with gender and AAT lung disease. Statistical analyses were performed using SPSS software (Version 21.0.0.0, Armonk, NY, US), and p-values of ≤0.05 were considered statistically significant.
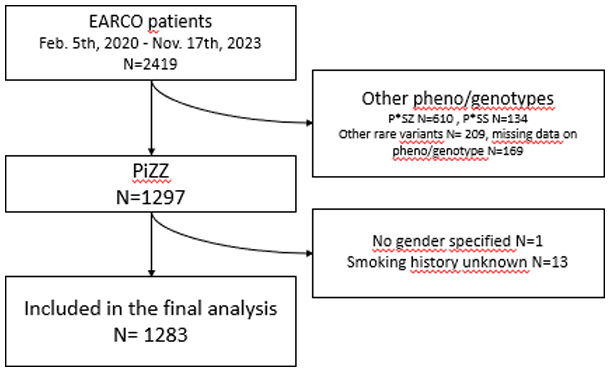
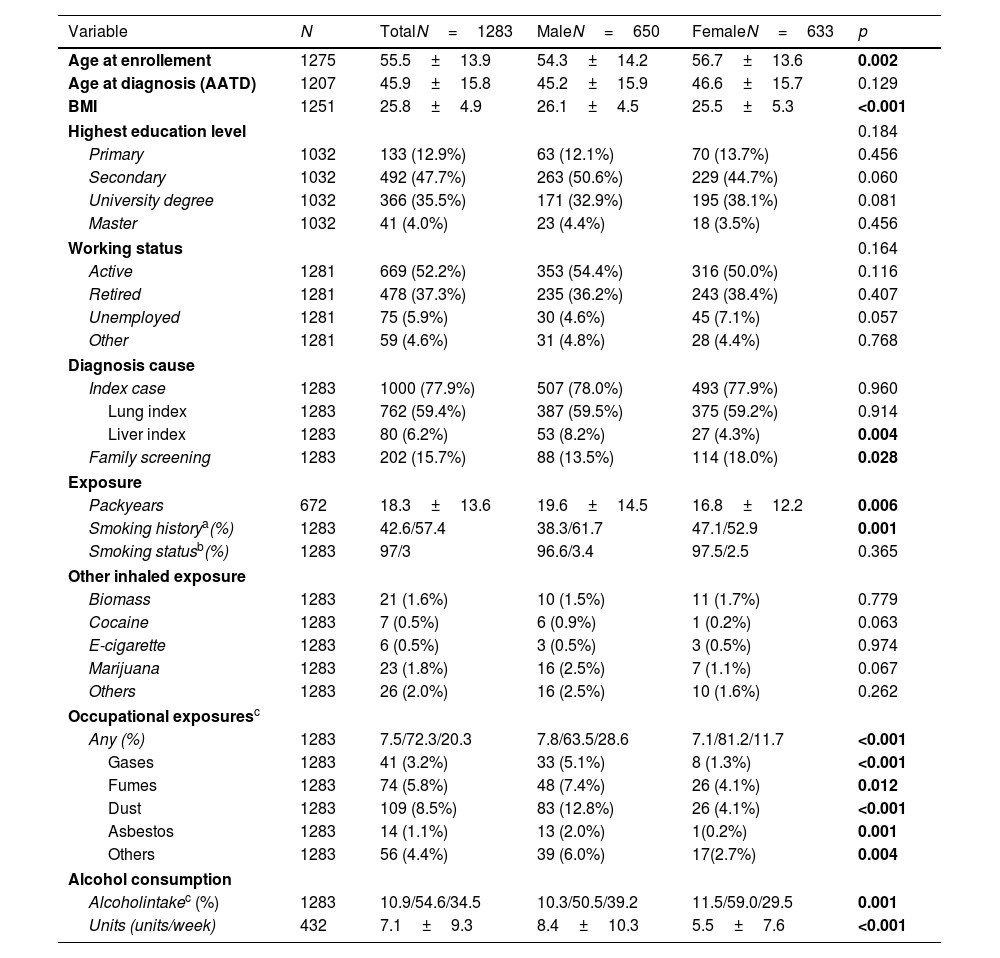
ResultsPatient characteristicsThe current study examined 1283 P*ZZ individuals with complete information on sex and smoking behaviour who were enrolled in the register between 05/02/2020 and 17/11/2023 (see flowchart in Supplementary Fig. S1). The sex ratio in the study population was relatively balanced, with 633 women and 650 men. Women were slightly older than men at enrolment, but not at AATD diagnosis, and had a lower BMI. Women were also more likely to have been diagnosed with the disease as part of a family screening and less likely to have been presented with liver disease. There were no differences between men and women in terms of employment (employed, retired, unemployed or other).
Compared to the male study participants, females were more likely to be never smokers (47.1% versus 38.3%, p<0.001) or reported a lower number of pack-years (16.8±12.2 versus 19.6±14.5, p=0.006). Exposure to other inhalable substances such as biomass, cocaine, e-cigarettes, marijuana or others was rare and did not show significant differences between the sexes. When occupational exposure was considered, there were significant differences between the sexes: men were significantly more likely to be exposed to gases (5.1 vs. 1.3%, p<0.001), fumes (7.4% vs. 4.1%, p=0.012), dust (12.8 vs. 4.1%, p<0.001), asbestos (2.0 vs. 0.2%, p=0.001) or other (6.0 vs. 2.7%, p=0.004) than women. There were also differences in alcohol consumption: women were less likely to report active alcohol consumption (29.5% versus 39.2%, p<0.001), and the number of alcohol units per week was lower (5.5±7.6 versus 8.4±10.3, p<0.001) (Table 1).
Demographics and exposure stratified for sex.
| Variable | N | TotalN=1283 | MaleN=650 | FemaleN=633 | p |
|---|---|---|---|---|---|
| Age at enrollement | 1275 | 55.5±13.9 | 54.3±14.2 | 56.7±13.6 | 0.002 |
| Age at diagnosis (AATD) | 1207 | 45.9±15.8 | 45.2±15.9 | 46.6±15.7 | 0.129 |
| BMI | 1251 | 25.8±4.9 | 26.1±4.5 | 25.5±5.3 | <0.001 |
| Highest education level | 0.184 | ||||
| Primary | 1032 | 133 (12.9%) | 63 (12.1%) | 70 (13.7%) | 0.456 |
| Secondary | 1032 | 492 (47.7%) | 263 (50.6%) | 229 (44.7%) | 0.060 |
| University degree | 1032 | 366 (35.5%) | 171 (32.9%) | 195 (38.1%) | 0.081 |
| Master | 1032 | 41 (4.0%) | 23 (4.4%) | 18 (3.5%) | 0.456 |
| Working status | 0.164 | ||||
| Active | 1281 | 669 (52.2%) | 353 (54.4%) | 316 (50.0%) | 0.116 |
| Retired | 1281 | 478 (37.3%) | 235 (36.2%) | 243 (38.4%) | 0.407 |
| Unemployed | 1281 | 75 (5.9%) | 30 (4.6%) | 45 (7.1%) | 0.057 |
| Other | 1281 | 59 (4.6%) | 31 (4.8%) | 28 (4.4%) | 0.768 |
| Diagnosis cause | |||||
| Index case | 1283 | 1000 (77.9%) | 507 (78.0%) | 493 (77.9%) | 0.960 |
| Lung index | 1283 | 762 (59.4%) | 387 (59.5%) | 375 (59.2%) | 0.914 |
| Liver index | 1283 | 80 (6.2%) | 53 (8.2%) | 27 (4.3%) | 0.004 |
| Family screening | 1283 | 202 (15.7%) | 88 (13.5%) | 114 (18.0%) | 0.028 |
| Exposure | |||||
| Packyears | 672 | 18.3±13.6 | 19.6±14.5 | 16.8±12.2 | 0.006 |
| Smoking historya(%) | 1283 | 42.6/57.4 | 38.3/61.7 | 47.1/52.9 | 0.001 |
| Smoking statusb(%) | 1283 | 97/3 | 96.6/3.4 | 97.5/2.5 | 0.365 |
| Other inhaled exposure | |||||
| Biomass | 1283 | 21 (1.6%) | 10 (1.5%) | 11 (1.7%) | 0.779 |
| Cocaine | 1283 | 7 (0.5%) | 6 (0.9%) | 1 (0.2%) | 0.063 |
| E-cigarette | 1283 | 6 (0.5%) | 3 (0.5%) | 3 (0.5%) | 0.974 |
| Marijuana | 1283 | 23 (1.8%) | 16 (2.5%) | 7 (1.1%) | 0.067 |
| Others | 1283 | 26 (2.0%) | 16 (2.5%) | 10 (1.6%) | 0.262 |
| Occupational exposuresc | |||||
| Any (%) | 1283 | 7.5/72.3/20.3 | 7.8/63.5/28.6 | 7.1/81.2/11.7 | <0.001 |
| Gases | 1283 | 41 (3.2%) | 33 (5.1%) | 8 (1.3%) | <0.001 |
| Fumes | 1283 | 74 (5.8%) | 48 (7.4%) | 26 (4.1%) | 0.012 |
| Dust | 1283 | 109 (8.5%) | 83 (12.8%) | 26 (4.1%) | <0.001 |
| Asbestos | 1283 | 14 (1.1%) | 13 (2.0%) | 1(0.2%) | 0.001 |
| Others | 1283 | 56 (4.4%) | 39 (6.0%) | 17(2.7%) | 0.004 |
| Alcohol consumption | |||||
| Alcoholintakec (%) | 1283 | 10.9/54.6/34.5 | 10.3/50.5/39.2 | 11.5/59.0/29.5 | 0.001 |
| Units (units/week) | 432 | 7.1±9.3 | 8.4±10.3 | 5.5±7.6 | <0.001 |
Abbreviations: BMI: body mass index; AAT: alpha 1 antitrypsin.
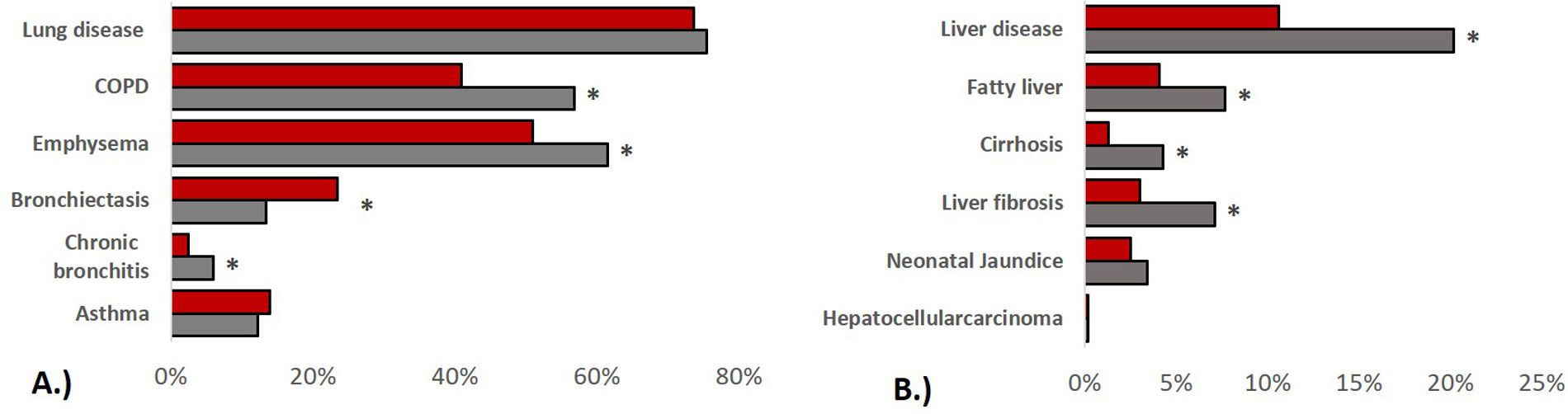
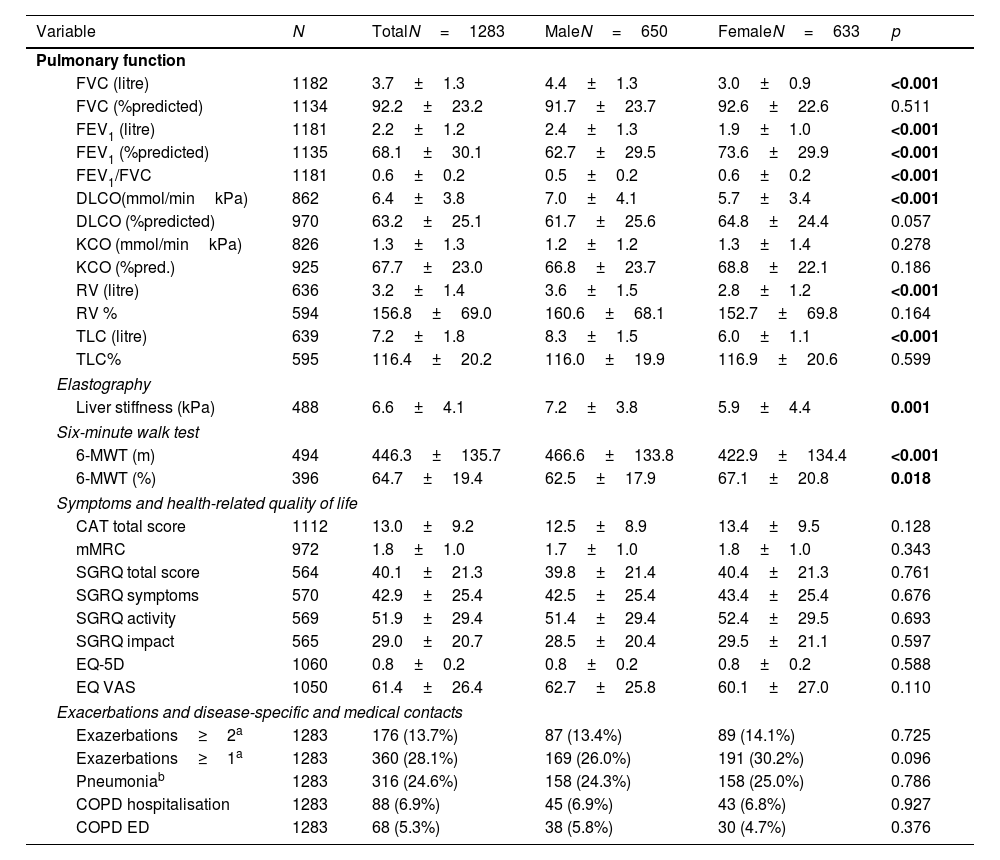
In terms of pulmonary AATD manifestations, women were less likely to have COPD (40.9% vs. 56.8%, p<0.001), emphysema (51.0% vs. 61.5%, p<0.001) or chronic bronchitis (2.5% vs. 6.0%, p=0.002), while they had a higher prevalence of bronchiectasis (23.5% vs. 13.4%, p<0.001). Corresponding to these medical diagnoses, the men showed a higher degree of airflow obstruction and a greater impairment of diffusion capacity in pulmonary function tests. In absolute values, the 6-min walking distance of women was shorter than that of men (422.9±134.4 vs. 466.6±133.8, p<0.001) although in relation to the corresponding reference values, men had a shorter walking distance (62.5±17.9%pred. vs. 67.1±20.8, p=0.018). In terms of liver involvement, male participants were more likely to have liver disease (20.2% vs.10.6%, p<0.001), liver cirrhosis (4.3% vs. 1.3%, p=0.001), liver fibrosis (7.1% vs. 3.0%, p=0.001) and fatty liver (7.7% vs. 4.1%, p=0.007). Patients characteristics are listed in Table 2 and Fig. 1.
Functional diagnostics, symptoms and disease-specific and medical contacts stratified for sex.
| Variable | N | TotalN=1283 | MaleN=650 | FemaleN=633 | p |
|---|---|---|---|---|---|
| Pulmonary function | |||||
| FVC (litre) | 1182 | 3.7±1.3 | 4.4±1.3 | 3.0±0.9 | <0.001 |
| FVC (%predicted) | 1134 | 92.2±23.2 | 91.7±23.7 | 92.6±22.6 | 0.511 |
| FEV1 (litre) | 1181 | 2.2±1.2 | 2.4±1.3 | 1.9±1.0 | <0.001 |
| FEV1 (%predicted) | 1135 | 68.1±30.1 | 62.7±29.5 | 73.6±29.9 | <0.001 |
| FEV1/FVC | 1181 | 0.6±0.2 | 0.5±0.2 | 0.6±0.2 | <0.001 |
| DLCO(mmol/minkPa) | 862 | 6.4±3.8 | 7.0±4.1 | 5.7±3.4 | <0.001 |
| DLCO (%predicted) | 970 | 63.2±25.1 | 61.7±25.6 | 64.8±24.4 | 0.057 |
| KCO (mmol/minkPa) | 826 | 1.3±1.3 | 1.2±1.2 | 1.3±1.4 | 0.278 |
| KCO (%pred.) | 925 | 67.7±23.0 | 66.8±23.7 | 68.8±22.1 | 0.186 |
| RV (litre) | 636 | 3.2±1.4 | 3.6±1.5 | 2.8±1.2 | <0.001 |
| RV % | 594 | 156.8±69.0 | 160.6±68.1 | 152.7±69.8 | 0.164 |
| TLC (litre) | 639 | 7.2±1.8 | 8.3±1.5 | 6.0±1.1 | <0.001 |
| TLC% | 595 | 116.4±20.2 | 116.0±19.9 | 116.9±20.6 | 0.599 |
| Elastography | |||||
| Liver stiffness (kPa) | 488 | 6.6±4.1 | 7.2±3.8 | 5.9±4.4 | 0.001 |
| Six-minute walk test | |||||
| 6-MWT (m) | 494 | 446.3±135.7 | 466.6±133.8 | 422.9±134.4 | <0.001 |
| 6-MWT (%) | 396 | 64.7±19.4 | 62.5±17.9 | 67.1±20.8 | 0.018 |
| Symptoms and health-related quality of life | |||||
| CAT total score | 1112 | 13.0±9.2 | 12.5±8.9 | 13.4±9.5 | 0.128 |
| mMRC | 972 | 1.8±1.0 | 1.7±1.0 | 1.8±1.0 | 0.343 |
| SGRQ total score | 564 | 40.1±21.3 | 39.8±21.4 | 40.4±21.3 | 0.761 |
| SGRQ symptoms | 570 | 42.9±25.4 | 42.5±25.4 | 43.4±25.4 | 0.676 |
| SGRQ activity | 569 | 51.9±29.4 | 51.4±29.4 | 52.4±29.5 | 0.693 |
| SGRQ impact | 565 | 29.0±20.7 | 28.5±20.4 | 29.5±21.1 | 0.597 |
| EQ-5D | 1060 | 0.8±0.2 | 0.8±0.2 | 0.8±0.2 | 0.588 |
| EQ VAS | 1050 | 61.4±26.4 | 62.7±25.8 | 60.1±27.0 | 0.110 |
| Exacerbations and disease-specific and medical contacts | |||||
| Exazerbations≥2a | 1283 | 176 (13.7%) | 87 (13.4%) | 89 (14.1%) | 0.725 |
| Exazerbations≥1a | 1283 | 360 (28.1%) | 169 (26.0%) | 191 (30.2%) | 0.096 |
| Pneumoniab | 1283 | 316 (24.6%) | 158 (24.3%) | 158 (25.0%) | 0.786 |
| COPD hospitalisation | 1283 | 88 (6.9%) | 45 (6.9%) | 43 (6.8%) | 0.927 |
| COPD ED | 1283 | 68 (5.3%) | 38 (5.8%) | 30 (4.7%) | 0.376 |
Abbreviations: FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; FEV1/FVC: Tiffeneau index; TLCO: transfer factor of the lung for carbon monoxide (CO); KCO: carbon monoxide transfer coefficient; RV: residual volume; TLC: total lung capacity; 6-MWD: six-minute walk test; CAT: COPD assessment test; mMRC: modified Medical Research Council Dyspnoea Scale; SGRQ: St. Georges Respiratory Questionnaire; EQ-5D: European Quality of Life 5 Dimensions 3 Level Version; EQ VAS: EuroQol visual analogue scale; COPD ED: COPD related emergency department visit.
Prevalence of lung and liver disease stratified by sex. (A): pulmonary disease, (B): liver disease, bars refer to indicated medical diagnoses, categorised by men (grey) and women (red), group comparisons were made using Chi-squared tests, p values<0.005 were considered statistically significant.
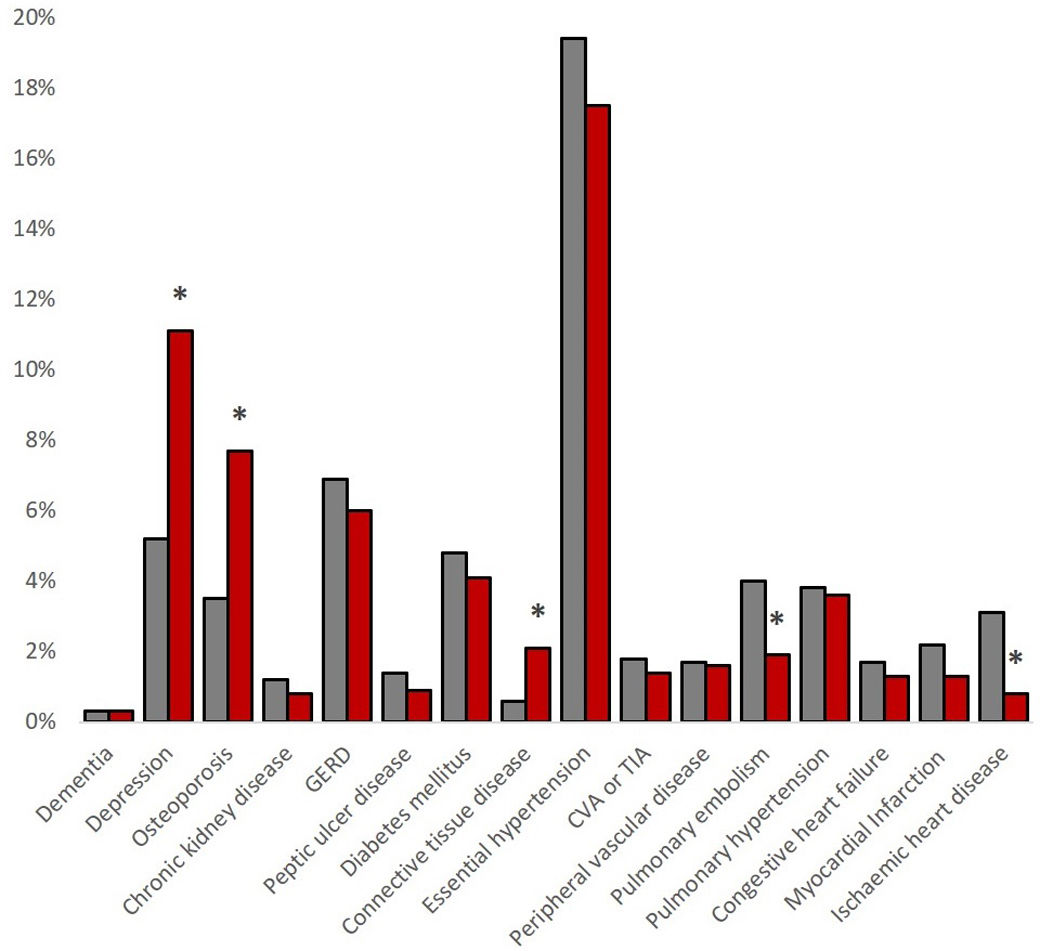
Regarding comorbidities, women reported lower rates of ischaemic heart disease (0.8% vs. 3.1%, p=0.003) and pulmonary embolism (1.9% vs. 4.0%, p=0.026), but higher rates of connective tissue diseases (2.1% vs. 0.6%, p=0.024), osteoporosis (7.7% vs. 3.5%, p=0.001) and depression (11.1% vs.5.2%, p<0.001). The sum of comorbidities was higher in men with a Charlson Index score of 1.2±1.3 vs. 0.9±1.7 in women (p<0.001), see Fig. 2.
Symptoms and quality of lifeOverall, when asked about their symptoms, women in the cohort reported the same symptom burden as men on the modified Medical Research Council Dyspnoea Scale (mMRC) and the COPD assessment test (CAT). With regard to health-related quality of life, there were no differences in the St. George's Respiratory Questionnaire (SGRQ) and in the European Quality of Life 5 Dimensions 3 Level Version (EQ5D), see Table 2.
Exacerbations and disease-specific and medical contactsIn the overall group, 28.1% of study participants reported at least one exacerbation in the previous year, 14% reported two or more exacerbations, with no gender differences. There were no gender-specific differences in outpatient emergency contacts due to pneumonia or COPD or in hospital admissions due to COPD), see Table 2.
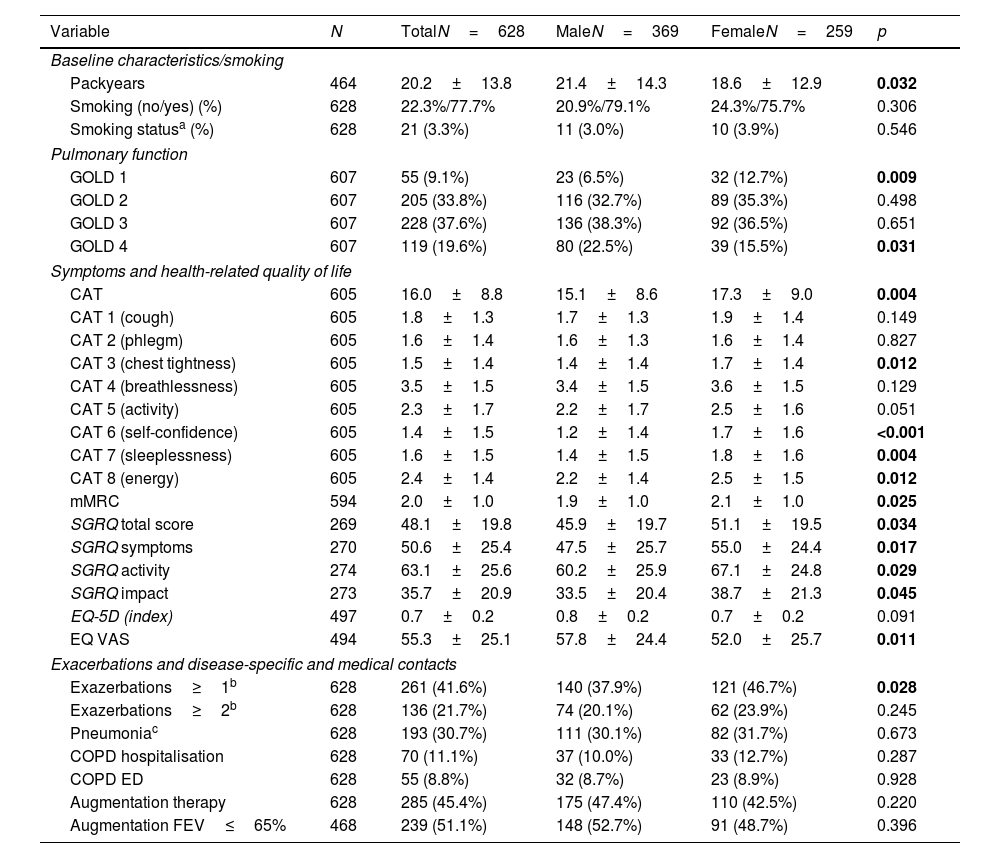
COPD subgroupGiven that women were less likely to be diagnosed with COPD, additional analyses were performed in the subgroup of 628 patients, diagnosed with COPD. The women with COPD had a lower number of PY than the men with COPD (18.6±12.9 vs.21.4±14.3, p=0.032).
In terms of pulmonary function, women had better FEV1pred. values and were more often in GOLD grade 1 and less in GOLD 4 than men. There were no differences in residual volumes and diffusion capacity. However, women with COPD had a significantly higher symptom burden. This was demonstrated by the CAT and the mMRC, where women had significantly higher scores than men (17.3±9.0 vs. 15.1±8.6, p=0.004 and 2.1±1.0 vs. 1.9±1.0, p=0.025). Women scored significantly higher than men, particularly on CAT items 3 (chest tightness), 6 (self-confidence), 7 (sleeplessness) and 8 (energy). The higher symptom burden was also reflected in a higher SGRQ total score, which was due to increased scores in all SGRQ sub-domains (p<0.05 for each). Women with COPD tended to have lower EQ-5D scores (0.8±0.2 vs. 0.7±0.2, p=0.091), indicating a poorer health status, but these values did not reach statistical significance. The EQ VAS were significantly lower in women (52.0±25.7 vs. 57.8±24.4, p=0.011). In addition, women in this subgroup were significantly more likely to report one or more exacerbations in the last year than men (46.7% vs. 37.9%, p=0.028), see Table 3.
COPD subgroup, pulmonary function, symptoms and disease-specific and medical contacts.
| Variable | N | TotalN=628 | MaleN=369 | FemaleN=259 | p |
|---|---|---|---|---|---|
| Baseline characteristics/smoking | |||||
| Packyears | 464 | 20.2±13.8 | 21.4±14.3 | 18.6±12.9 | 0.032 |
| Smoking (no/yes) (%) | 628 | 22.3%/77.7% | 20.9%/79.1% | 24.3%/75.7% | 0.306 |
| Smoking statusa (%) | 628 | 21 (3.3%) | 11 (3.0%) | 10 (3.9%) | 0.546 |
| Pulmonary function | |||||
| GOLD 1 | 607 | 55 (9.1%) | 23 (6.5%) | 32 (12.7%) | 0.009 |
| GOLD 2 | 607 | 205 (33.8%) | 116 (32.7%) | 89 (35.3%) | 0.498 |
| GOLD 3 | 607 | 228 (37.6%) | 136 (38.3%) | 92 (36.5%) | 0.651 |
| GOLD 4 | 607 | 119 (19.6%) | 80 (22.5%) | 39 (15.5%) | 0.031 |
| Symptoms and health-related quality of life | |||||
| CAT | 605 | 16.0±8.8 | 15.1±8.6 | 17.3±9.0 | 0.004 |
| CAT 1 (cough) | 605 | 1.8±1.3 | 1.7±1.3 | 1.9±1.4 | 0.149 |
| CAT 2 (phlegm) | 605 | 1.6±1.4 | 1.6±1.3 | 1.6±1.4 | 0.827 |
| CAT 3 (chest tightness) | 605 | 1.5±1.4 | 1.4±1.4 | 1.7±1.4 | 0.012 |
| CAT 4 (breathlessness) | 605 | 3.5±1.5 | 3.4±1.5 | 3.6±1.5 | 0.129 |
| CAT 5 (activity) | 605 | 2.3±1.7 | 2.2±1.7 | 2.5±1.6 | 0.051 |
| CAT 6 (self-confidence) | 605 | 1.4±1.5 | 1.2±1.4 | 1.7±1.6 | <0.001 |
| CAT 7 (sleeplessness) | 605 | 1.6±1.5 | 1.4±1.5 | 1.8±1.6 | 0.004 |
| CAT 8 (energy) | 605 | 2.4±1.4 | 2.2±1.4 | 2.5±1.5 | 0.012 |
| mMRC | 594 | 2.0±1.0 | 1.9±1.0 | 2.1±1.0 | 0.025 |
| SGRQ total score | 269 | 48.1±19.8 | 45.9±19.7 | 51.1±19.5 | 0.034 |
| SGRQ symptoms | 270 | 50.6±25.4 | 47.5±25.7 | 55.0±24.4 | 0.017 |
| SGRQ activity | 274 | 63.1±25.6 | 60.2±25.9 | 67.1±24.8 | 0.029 |
| SGRQ impact | 273 | 35.7±20.9 | 33.5±20.4 | 38.7±21.3 | 0.045 |
| EQ-5D (index) | 497 | 0.7±0.2 | 0.8±0.2 | 0.7±0.2 | 0.091 |
| EQ VAS | 494 | 55.3±25.1 | 57.8±24.4 | 52.0±25.7 | 0.011 |
| Exacerbations and disease-specific and medical contacts | |||||
| Exazerbations≥1b | 628 | 261 (41.6%) | 140 (37.9%) | 121 (46.7%) | 0.028 |
| Exazerbations≥2b | 628 | 136 (21.7%) | 74 (20.1%) | 62 (23.9%) | 0.245 |
| Pneumoniac | 628 | 193 (30.7%) | 111 (30.1%) | 82 (31.7%) | 0.673 |
| COPD hospitalisation | 628 | 70 (11.1%) | 37 (10.0%) | 33 (12.7%) | 0.287 |
| COPD ED | 628 | 55 (8.8%) | 32 (8.7%) | 23 (8.9%) | 0.928 |
| Augmentation therapy | 628 | 285 (45.4%) | 175 (47.4%) | 110 (42.5%) | 0.220 |
| Augmentation FEV≤65% | 468 | 239 (51.1%) | 148 (52.7%) | 91 (48.7%) | 0.396 |
Abbreviations: a(nonsmoker/current smoker); CAT: COPD assessment test; mMRC: modified Medical Research Council Dyspnoea Scale; SGRQ: St. Georges Respiratory Questionnaire; EQ-5D: European Quality of Life 5 Dimensions 3 Level Version; EQ VAS: EuroQol visual analogue scale; COPD ED: COPD related emergency department visits btotal in the last 12 months cambulatory in the last 12 months.
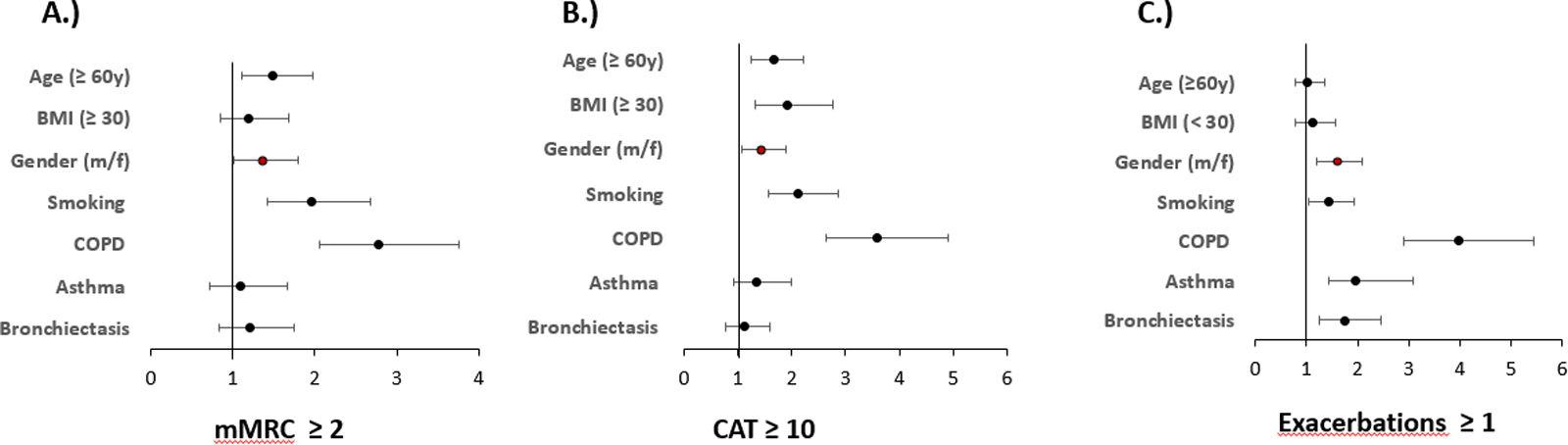
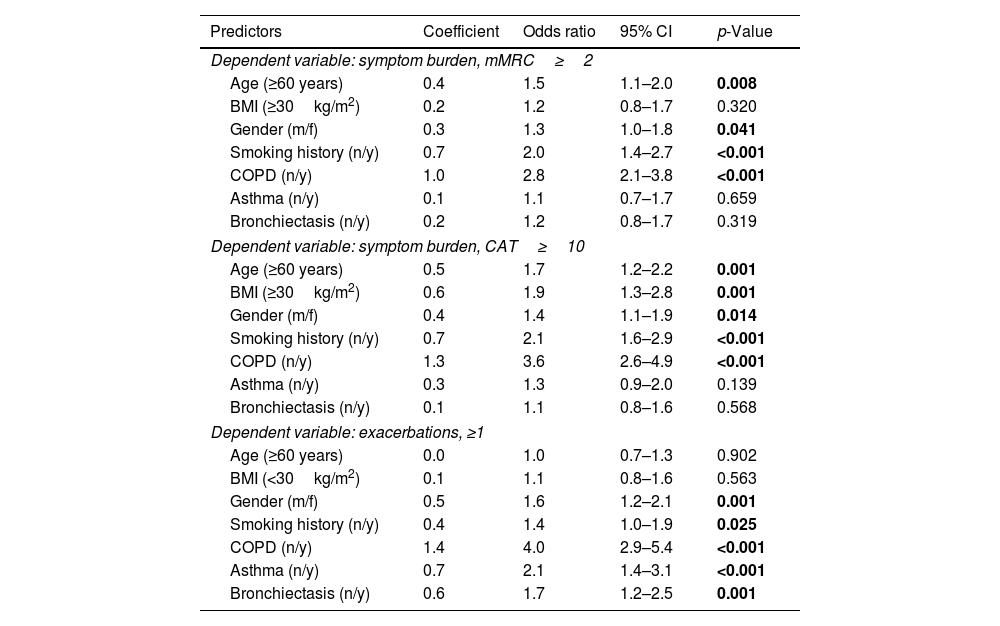
Multivariate regression analyses of the outcome variables mMRC (≥2) or CAT (≥10) to record symptom burden and exacerbations (≥1 in the previous year), always taking into account age, gender, BMI and smoking status as well as the presence of COPD, asthma or bronchiectasis, showed that sex is an independent predictor of the aforementioned outcomes in all three cases. See Fig. 3 and Table 4.
Predictors of symptoms and exacerbations. The figure shows the odds ratios and 95% confidence intervals for predictors of mMRC≥2, CAT≥10 binary and exacerbations≥1 derived from binary logistic regression analyses. Numerical values correspond to those in Table 4.
Independent predictors of symptoms and exacerbations Shown are the results of the logistic regression analyses for the outcomes; symptom burden, mMRC ≥2 and CAT ≥10 as well as axacerbations ≥1 in the previous year.
| Predictors | Coefficient | Odds ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Dependent variable: symptom burden, mMRC≥2 | ||||
| Age (≥60 years) | 0.4 | 1.5 | 1.1–2.0 | 0.008 |
| BMI (≥30kg/m2) | 0.2 | 1.2 | 0.8–1.7 | 0.320 |
| Gender (m/f) | 0.3 | 1.3 | 1.0–1.8 | 0.041 |
| Smoking history (n/y) | 0.7 | 2.0 | 1.4–2.7 | <0.001 |
| COPD (n/y) | 1.0 | 2.8 | 2.1–3.8 | <0.001 |
| Asthma (n/y) | 0.1 | 1.1 | 0.7–1.7 | 0.659 |
| Bronchiectasis (n/y) | 0.2 | 1.2 | 0.8–1.7 | 0.319 |
| Dependent variable: symptom burden, CAT≥10 | ||||
| Age (≥60 years) | 0.5 | 1.7 | 1.2–2.2 | 0.001 |
| BMI (≥30kg/m2) | 0.6 | 1.9 | 1.3–2.8 | 0.001 |
| Gender (m/f) | 0.4 | 1.4 | 1.1–1.9 | 0.014 |
| Smoking history (n/y) | 0.7 | 2.1 | 1.6–2.9 | <0.001 |
| COPD (n/y) | 1.3 | 3.6 | 2.6–4.9 | <0.001 |
| Asthma (n/y) | 0.3 | 1.3 | 0.9–2.0 | 0.139 |
| Bronchiectasis (n/y) | 0.1 | 1.1 | 0.8–1.6 | 0.568 |
| Dependent variable: exacerbations, ≥1 | ||||
| Age (≥60 years) | 0.0 | 1.0 | 0.7–1.3 | 0.902 |
| BMI (<30kg/m2) | 0.1 | 1.1 | 0.8–1.6 | 0.563 |
| Gender (m/f) | 0.5 | 1.6 | 1.2–2.1 | 0.001 |
| Smoking history (n/y) | 0.4 | 1.4 | 1.0–1.9 | 0.025 |
| COPD (n/y) | 1.4 | 4.0 | 2.9–5.4 | <0.001 |
| Asthma (n/y) | 0.7 | 2.1 | 1.4–3.1 | <0.001 |
| Bronchiectasis (n/y) | 0.6 | 1.7 | 1.2–2.5 | 0.001 |
Our analyses revealed several differences between the sexes, including differences in alcohol and tobacco consumption, occupational exposure to pollutants, the prevalence of AATD-associated lung and liver diseases, and the pattern of comorbidities. There were also significant differences in the quantity and quality of symptoms and the number of exacerbations reported, most evident in the subgroup of patients with COPD. Regardless of the type or presence of lung disease, female gender was associated with higher symptom burden as measured by the CAT or mMRC. Female sex was also an independent risk factor for exacerbations. In terms of risk behaviours, men in the registry were significantly more likely to be current or former smokers, to have a higher number of pack-years, and to report more frequent and higher daily alcohol consumption. This corresponds to the data from the Canadian AAT register, in which men also have a higher tobacco consumption.21 In addition, the men in the cohort were significantly more likely to have occupational exposure to pollutants.
Disease manifestations also differed between the sexes, with more men diagnosed with liver disease, chronic bronchitis, COPD or emphysema, and more women diagnosed with bronchiectasis. These differences can be partly, but not entirely, explained by differences in behaviour. Although men in the cohort had a higher tobacco consumption, this only amounted to about 3 pack-years overall, and a number of studies have shown that women are more susceptible to tobacco-related pulmonary harm than men. Sørheim et al. investigated sex differences in the effects of smoking on lung function in a population with early COPD and/or COPD development at low levels of tobacco exposure. Female sex was associated with a greater decline in lung function in both groups of patients.22 Data from two independent Danish population-based cohorts, the Copenhagen City Heart Study and the Glostrup Population Studies, with a total of 13,897 participants who were followed up for up to 16 years, came to a similar conclusion: smoking impairs lung function more in women than in men. When the number of pack years (PY) was taken into account, the female participants in the two Danish cohorts also had a higher risk of being hospitalised for COPD than the male participants.23 In addition, an analysis of the German AATD Registry did not show a clear gender dominance of any AATD phenotype, but it should be noted that the information in this registry is based on patient self-report and only asked about lung and liver disease in general.24 In the EARCO registry, data are entered by the study centres and the information on lung or liver disease is much more detailed.12,13 This made it possible to distinguish between different lung diseases. With regard to the various lung diseases, it is also surprising that although women are less likely to report a diagnosis of chronic bronchitis, they are more likely to suffer from bronchiectasis, as chronic bronchitis is usually a clinical diagnosis that should have a large overlap with the symptoms of bronchiectasis. In previous COPD studies, women were often less willing to report cough and sputum production.2,11 This could be due to the shame associated with traditional gender roles and definitions of femininity. Gender differences in bronchiectasis have been known for many years, with 60.9% of participants in the European Bronchiectasis registry (EMBARC) being female.25 In general, bronchiectasis is the endpoint of a wide range of diseases and the causes of these sex differences are poorly understood. Sex steroid hormones are thought to vary in type, pattern and concentration throughout life and between the sexes and may play a central role in various lung diseases as well as in sex differences in the prevalence of bronchiectasis.26
There was a clear predominance of men with liver disease in our cohort. In general, it should be noted that liver disease was less common in our cohort, which may be partly due to the fact that the EARCO study centres were predominantly pneumology departments, which introduces a certain selection bias. The observed sex differences are consistent with data from a large UK biobank study showing that male sex, obesity, diabetes and older age are associated with an increased risk of liver fibrosis/cirrhosis and primary liver cancer.27
There were some differences in other comorbidities between men and women in our cohort; overall, the rate of cardiovascular comorbidities was low, as previously described in other AATD cohorts.28,29 Men were more likely to have ischaemic heart disease or a history of pulmonary embolism, while women were more likely to have been diagnosed with osteoporosis or depression.
Another interesting finding was the difference in reported symptoms: although women were less likely to have COPD, they reported the same overall level of COPD-specific symptoms in the CAT and the mMRC. A recent study by EARCO comparing 629 P*ZZ individuals from Northern and Southern European countries showed relevant differences in the CAT score between the two groups.30 This highlights the fact that, in addition to the diagnosis itself, a large number of influencing factors play a role, which is why the investigation of such a question in a large international cohort such as EARCO is important. In our analyses, we also found relevant differences between the sexes in terms of the frequency of reported exacerbations. In the overall collective, the number of exacerbations was the same, which can be attributed to the fact that the subgroup of female COPD patients reported significantly more exacerbations than their male counterparts. Female COPD patients also had a significantly higher symptom burden compared to male patients. In the multivariate analyses conducted for the entire cohort, female sex was an independent predictor of exacerbations in the last year, along with age, BMI, smoking status and a diagnosis of COPD, asthma or bronchiectasis. In addition, female sex, together with age, BMI and a COPD diagnosis, was associated with higher symptom burden as measured by both the mMRC scale and the CAT score. The results correspond to data from the Spanish COPD cohort INSEPOC, analysing a sample of 4500 COPD patients. The women with COPD in the study were younger, smoked less and had better lung function. Nevertheless, these women reported a significantly poorer quality of life and more exacerbations.31 A pooled analysis of 17,139 patients from 22 COPD cohorts showed that certain disease characteristics were different in women than in men, including higher levels of self-reported dyspnoea and COPD exacerbations; interestingly, the long-term survival of women with COPD in this study appeared to be better than that of men.2 It remains to be seen whether this risk profile has an impact on the prognosis, e.g. with regard to the development of lung function in AATD.
LimitationsAlthough the study was conducted in a large international registry with high data quality, there are some limitations, including the fact that the pulmonary diagnoses (e.g. emphysema and bronchiectasis) as well as comorbidities recorded were based on previous medical diagnoses. In this respect, it may not have been possible to rule out sex-based ascertainment bias in diagnosis. Nevertheless, this is the first comprehensive international study of sex-specific aspects in AATD. We did not collect self-identified gender, only biological sex, so wider study of this in comparison to sex may also be relevant in future.
ConclusionOur analysis of sex and gender aspects in AATD using the well-characterised international AATD cohort EARCO has shown that sex is important in AATD in many ways. The differences in risk behaviours highlight the need for early diagnosis and for sex and to be taken into account in individualised tobacco and alcohol prevention programmes. The differences in the pulmonary manifestations of AATD, such as the high proportion of women with bronchiectasis or the differences in exacerbation frequency and symptom burden, demonstrate the need for tailored therapeutic approaches to the different aspects of AATD and that sex should be included in these considerations.
FundingThe International EARCO Registry is funded by unrestricted grants from Grifols, CSL Behring, Kamada, pH Pharma and Takeda to the European Respiratory Society (ERS).
AMT is funded by the NIHR Midlands Patient Safety Research Collaboration (PSRC) and Applied Research Collaborative West Midlands. She has also received grant funding from NIHR HTA and EME. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Authors’ contributionsFCT and HE drafting of the manuscript. FCT and HE performed the statistical analysis. All authors participated in the study concept, design and acquisition of data. All authors performed a critical revision and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Conflict of interestHilal Ersöz has received support for attending meetings and/or travel from CSL Behring. María Torres-Durán has received consulting fees from Grifols, speaker fees from CSL Behring, GSK and Chiesi, support for attending meetings and/or travel from Chiesi and Faes. Carlota Rodríguez García has received speaker fees from Glaxo-SmithKline, Grifols and Chiesi, support for attending meetings and/or travel from Grifols, Chiesi and Cipla. José Luis López-Campos has received grants from Menarini and Grifols, consulting fees from GlaxoSmithKline, CSL Behring, Bial, Gebro, Boehringer and AstraZeneca, speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, CSL Behring, Faes, Gebro, GlaxoSmithKline, Grifols, Zambon and Menarini, support for attending meetings and/or travel from Boehringer, GSK, Menarini, Chiesi, CSL Behring and Grifols. Christian F. Clarenbach has received consulting fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Daiichi Synkyo, GlaxoSmithKline, Novartis, Sanofi, OM Pharma, MSD, Grifols and Vifor and speaker fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Daiichi Synkyo, GlaxoSmithKline, Novartis, Sanofi, OM Pharma, MSD, Grifols and Vifor. Kenneth R. Chapman has received grants from Astra Zeneca, BMS, Grifols, Sanofi, Bellus, GlaxoSmithKline, Roche, Regeneron and Takeda, consulting fees from GlaxoSmithKline, Inhibrx, Roche,Takeda, Grifols, Regeneron and Sanofi, speaker fees from AstraZeneca, Grifols, Novartis, Takeda, Boehringer Ingelheim, GlaxoSmithKline, Regeron, Valeo and has a leadership in AlphaNet Canada. José María Hernández-Pérez has received payment of consumables within the research project from Grifols and CSL Behring. Catarina Guimarães has received speaker fees from CSL Behring. Timm Greulich has received grants from Grifols and consulting and speaker fees and support for attending meetings and/or travel from CSL-Behring and Grifols. Miriam Barrecheguren has received consulting fees from GSK, Novartis, Chiesi, CSL Behring, Bial and Boehringer Ingelheim, speaker fees from Grifols, Menarini, CSL Behring, GSK, Boehringer Ingelheim, and Chiesi, support for attending meetings and/or travel from Chiesi and CSL Behring. Andreas Rembert Koczulla has received grants from Grifols, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, CSL Behring, Berlin Chemie, Novartis,Pfizer, PulmonX, Sanofi, Resmed and Grifols, speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Kamada, CSL Behring, Grifols and Novartis and support for attending meetings and/or travel from Boehringer Ingelheim, Grifols, CSL. Philipp Höger has received speaker fees from Grifols. Franziska C. Trudzinski reports payment or honoraria for lectures, or reimbursement of travel expenses from Novartis AG, GlaxoSmithKline, Chiesi, Boehringer Ingelheim GmbH, Grifols, Streamed up, RG Gesellschaft für Information und Organisation mbH and AstraZeneca. Hanan Tanash, Angelo Guido Corsico, Eva Bartošovská, Arturo Olivares Rivera and Felix Herth have no competing interests to declare.
The authors would like to thank the patients who participated in this study and the EARCO study investigators (listed below). We wish to acknowledge Elise Heuvelin and Valerija Arsovski (European Respiratory Society, Lausanne, Switzerland) for their support in the management of EARCO, and Helena Miquel (Bioclever, Barcelona, Spain) for their support in EARCO data monitoring.
Mariano Fernández-Acquier, Andrés Echazarreta (Argentina); Georg-Christian Funk (Austria); Wim Janssens, Silvia Pérez-Bogerd, Eric Derom (Belgium); Ken Chapman (Canada); Leidy Prada (Colombia); Ana Hecimovic (Croatia); Eva Bartosovska, (Czech Republic); Alan Altraja, Jaanus Martti (Estonia); Jaens-Ulrik Jensen (Denmark); Maeva Zysman, Jean-François Mornex, Hakima Ouksel, Martine Louise Reynaud-Gaubert, Vincent Bunel-Gourdy (France); Timm Greulich, Franziska Trudzinski, Rembert Koczulla, Matthias Welsner (Germany); Gerry McElvaney (Ireland); Angelo G. Corsico, Ilaria Ferrarotti, Simone Scarlata, Mario Malerba (Italy); Jan Stolk, Emily F. van’t Wout (the Netherlands); Joanna Chorowstoska-Wyminko (Poland); Catarina Guimaraes, Maria Sucena, Ana Caldas, Raquel Marçoa, Isabel Ruivo dos Santos, Bebiana Conde, Maria Joana Reis Amado, Maia Da Silva, Teresa Martin, Rita Boaventura, Cristina Santos, Sonia Isabel Silva Guerra, Filipa Costa, Joana Gomes, Gabriela Santos (Portugal); Ruxandra Ulmeanu (Romania); Maja Omcikus (Serbia); María Torres-Duran, Marc Miravitlles, Miriam Barrecheguren, Juan Luis Rodriguez-Hermosa, Myriam Calle-Rubio, José María Hernández-Pérez, José Luis López-Campos, Francisco Casas-Maldonado, Ana Bustamante, Carlota Rodriguez-García, Marta García-Clemente, Cruz González, Eva Tabernero, Lourdes Lázaro, Virginia Almadana, Nuria Rodriguez-Lázaro, Mar Fernández-Nieto, Francisco Javier Michel de la Rosa, Carlos Martínez-Rivera, Layla Diab, María Isabel Parra, Rosanel Amaro, Ramón Tubío (Spain); Hanan Tanash, Eeva Piitulainen (Sweden); Christian F. Clarenbach (Switzerland); Serap Argun Baris, Dilek Karadogan, Genç Sebahat (Turkey); Alice M. Turner, Beatriz Lara, David G. Parr, Charlotte Bolton, John Hurst, Ravi Mahadeva, Nicholas Hopkinson, Bibek Gooptu (UK). EARCO Steering committee: Christian F. Clarenbach and Marc Miravitlles (co-chairs), Karen O’Hara, Marion Wilkens, Alice M. Turner, David G. Parr, Angelo Corsico, Catarina Guimaraes, Hanan Tanash. Scientific advisory Board: Robert Bals, Jan Stolk, Joanna Chorostowska-Wynimko, Ilaria Ferrarotti, Gerry McElvaney and Robert A. Stockley.