The Global Lung Function Initiative (GLI) has proposed new criteria for airflow limitation (AL) and recommends using these to interpret spirometry. The objective of this study was to explore the impact of the application of the AL GLI criteria in two well characterized GOLD-defined COPD cohorts.
MethodsCOPD patients from the BODE (n=360) and the COPD History Assessment In SpaiN (CHAIN) cohorts (n=722) were enrolled and followed. Age, gender, pack-years history, BMI, dyspnea, lung function measurements, exercise capacity, BODE index, history of exacerbations and survival were recorded. CT-detected comorbidities were registered in the BODE cohort. The proportion of subjects without AL by GLI criteria was determined in each cohort. The clinical, CT-detected comorbidity, and overall survival of these patients were evaluated.
ResultsIn total, 18% of the BODE and 15% of the CHAIN cohort did not meet GLI AL criteria. In the BODE and CHAIN cohorts respectively, these patients had a high clinical burden (BODE≥3: 9% and 20%; mMRC≥2: 16% and 45%; exacerbations in the previous year: 31% and 9%; 6MWD<350m: 15% and 19%, respectively), and a similar prevalence of CT-diagnosed comorbidities compared with those with GLI AL. They also had a higher rate of long-term mortality – 33% and 22% respectively.
ConclusionsAn important proportion of patients from 2 GOLD-defined COPD cohorts did not meet GLI AL criteria at enrolment, although they had a significant burden of disease. Caution must be taken when applying the GLI AL criteria in clinical practice.
In 2012, the European Respiratory Society (ERS) Task Force of the Global Lung Function Initiative (GLI) published multi-ethnic reference values for spirometry for the 3–95-year age range. The report included a new proposal to define airflow limitation (AL) based on the lower limit of normal (LLN) values of FEV1/FVC.1 The same authors (2) later recognized that adoption of the GLI 2022 equations could have “smalleffects” on the detection of obstructive ventilatory defects, and also recognized that “while the LLN methodology is based on an appropriate statistical approach, the clinical validity of either approach hinges on evidence of respiratory disease being present in those subjects who meet the respective inclusioncriteria”.2
Although GLI recommends the immediate adoption of these equations for spirometry interpretation in clinical practice,3 little is known about the potential impact in patients with well characterized clinical chronic obstructive pulmonary disease (COPD) defined by the fixed ratio (post bronchodilator FEV1/FVC<0.70), an easily calculated threshold value that predicts poor outcomes including mortality in different populations and cohorts worldwide.4–6
We hypothesized that the GLI criteria could misclassify a significant proportion of patients with clinical COPD who present significant limitations, such as dyspnea, exacerbations, limited exercise capacity, associated comorbidities, and compromised survival. To test this hypothesis, we applied the GLI AL criteria to 2 well characterized COPD cohorts who met spirometric AL criteria as defined by the Global Obstructive Lung Disease (GOLD).7 In the group of subjects who did not reach the threshold for airflow obstruction defined by GLI, we determined the prevalence of important clinical and radiological outcomes and determined their 7-year mortality.
MethodsThis was an observational analysis of outcomes in 2 COPD cohorts prospectively recruited between 1995 and 2011 in 12 pulmonary clinics located in tertiary university hospitals in Spain and followed yearly for an average of 7 years. The BODE and the COPD History Assessment In SpaiN (CHAIN) studies used a similar methodology, and the characteristics of both studies have been published elsewhere.4,8 In brief, they prospectively enrolled patients attending clinics and then followed them over time. COPD was defined according to the GOLD guidelines by the presence of symptoms compatible with the disease, smoking history≥10 pack-years, and a forced expiratory volume in 1second (FEV1)/forced vital capacity (FVC)<0.7 after 400μg of inhaled salbutamol.7 To participate in both studies, patients had to be stable for at least 8 weeks and be receiving optimal medical therapy according to current guidelines.7 All participants signed the informed consent form approved by the ethics committees (Comité de Etica de la Investigación, Hospital Universitario la Candelaria, Tenerife; 258/2009).
MeasurementsThe following baseline variables were recorded in all subjects: age, sex, body mass index (BMI) in kgm−2 and smoking history (pack years, smoking status). Spirometric values including FEV1/FVC were measured following the European Respiratory Society/American Thoracic Society (ERS/ATS) recommendations.9 Mean predicted values and Z-scores were derived using prediction equations from the European Community for Steel and Coal (ECSC)/ERS.10 The GLI 2012 calculations1 utilize mean predicted value, variation and skewness of the data to provide Z-scores and LLN specifically calculated for each individual. Airflow limitation was diagnosed if the FEV1/FVC was Z-score was <−1.6445. Subjects that did not meet these GLI criteria were deemed to have no AL and were selected for this analysis.
All subjects underwent a 6-min walking distance test performed according to ATS recommendations.11 The BODE index was calculated as previously reported.4 Exacerbations were defined by worsening of respiratory symptoms beyond normal daily variations that required the use of antibiotics, steroids or both, medical consultation or admission to hospital.7 In the CHAIN cohort, respiratory symptoms associated with quality of life were evaluated using the COPD Assessment Test (CAT).12 Comorbidities were rated using the Charlson index.13 Survival status was determined by direct follow-up, review of death certificates, or contact with family members if needed, and the methodology has been described elsewhere.4,8
Chest CT protocol and CT-assessed comorbiditiesAt baseline, subjects from the BODE cohort underwent a low-dose chest study using multidetector-row (16-detector or 64-detector) CT scans. The images were acquired at end-inspiration and extended from the thoracic inlet to the upper abdomen, with the patient in the supine position. The following parameters were used: 120kV, 40mA, 32mm×0.6mm detector collimation, pitch 1. Images were reconstructed with 5mm and 1mm slice thickness using soft tissue (B31f) and high-resolution (B60f) reconstruction algorithms to evaluate the mediastinum and lung parenchyma, respectively. CT scans were evaluated by 2 chest radiologists (AE and GB), blinded to clinical data. The methodology used to define each comorbidity identified on chest CT has been described elsewhere.14
Statistical analysisWe used the Kolmogorov–Smirnov test the variables for normal distribution. Categorical variables were summarized as relative frequencies and normally distributed variables were described as mean (standard deviation [SD]) (normally distributed variables were found). The Chi-square test was used to compare the percentage of CT-diagnosed comorbidities in subjects with and without GLI AL criteria. Kaplan–Meier survival analysis was used to test the independent association of each category (AL GLI criteria vs no AL GLI criteria) with all-cause survival adjusted by FEV1% of predicted according to GLI values. The log rank test was used to analyze differences between groups. Significance was established as a two-tailed p-value≤0.05. We used SPSS 26.0, Chicago, USA for the statistical analysis.
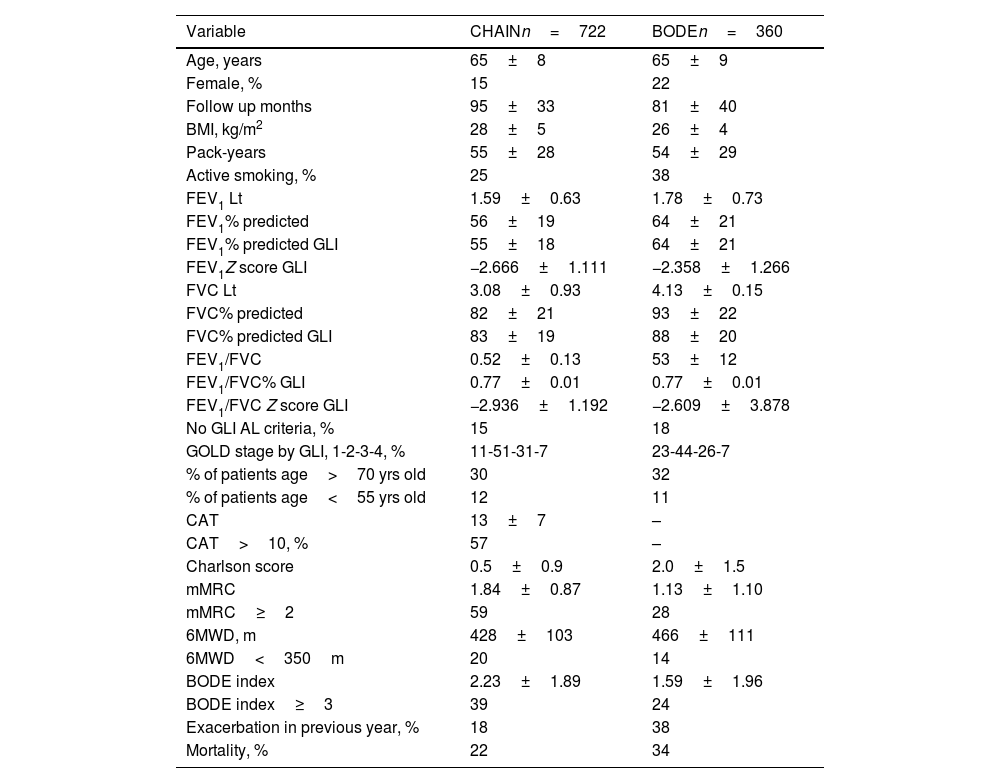
ResultsSeven hundred and twenty-two patients from the CHAIN cohort and 360 patients from the BODE cohort were included. Patient characteristics are shown in Table 1. Both cohorts were predominantly men, slightly overweight, with a similar smoking history, mostly in GOLD stages 1 and 2. Patients in the BODE cohort presented more comorbidities, but a higher proportion of patients in the CHAIN cohort presented mMRC≥2, 6MWD≤350m and BODE≥3. A significant proportion of patients (15% in the CHAIN cohort and 18% in the BODE cohort) did not meet the GLI criteria for AL.
Clinical and physiological characteristics of patients from each cohort.
| Variable | CHAINn=722 | BODEn=360 |
|---|---|---|
| Age, years | 65±8 | 65±9 |
| Female, % | 15 | 22 |
| Follow up months | 95±33 | 81±40 |
| BMI, kg/m2 | 28±5 | 26±4 |
| Pack-years | 55±28 | 54±29 |
| Active smoking, % | 25 | 38 |
| FEV1 Lt | 1.59±0.63 | 1.78±0.73 |
| FEV1% predicted | 56±19 | 64±21 |
| FEV1% predicted GLI | 55±18 | 64±21 |
| FEV1Z score GLI | −2.666±1.111 | −2.358±1.266 |
| FVC Lt | 3.08±0.93 | 4.13±0.15 |
| FVC% predicted | 82±21 | 93±22 |
| FVC% predicted GLI | 83±19 | 88±20 |
| FEV1/FVC | 0.52±0.13 | 53±12 |
| FEV1/FVC% GLI | 0.77±0.01 | 0.77±0.01 |
| FEV1/FVC Z score GLI | −2.936±1.192 | −2.609±3.878 |
| No GLI AL criteria, % | 15 | 18 |
| GOLD stage by GLI, 1-2-3-4, % | 11-51-31-7 | 23-44-26-7 |
| % of patients age>70 yrs old | 30 | 32 |
| % of patients age<55 yrs old | 12 | 11 |
| CAT | 13±7 | – |
| CAT>10, % | 57 | – |
| Charlson score | 0.5±0.9 | 2.0±1.5 |
| mMRC | 1.84±0.87 | 1.13±1.10 |
| mMRC≥2 | 59 | 28 |
| 6MWD, m | 428±103 | 466±111 |
| 6MWD<350m | 20 | 14 |
| BODE index | 2.23±1.89 | 1.59±1.96 |
| BODE index≥3 | 39 | 24 |
| Exacerbation in previous year, % | 18 | 38 |
| Mortality, % | 22 | 34 |
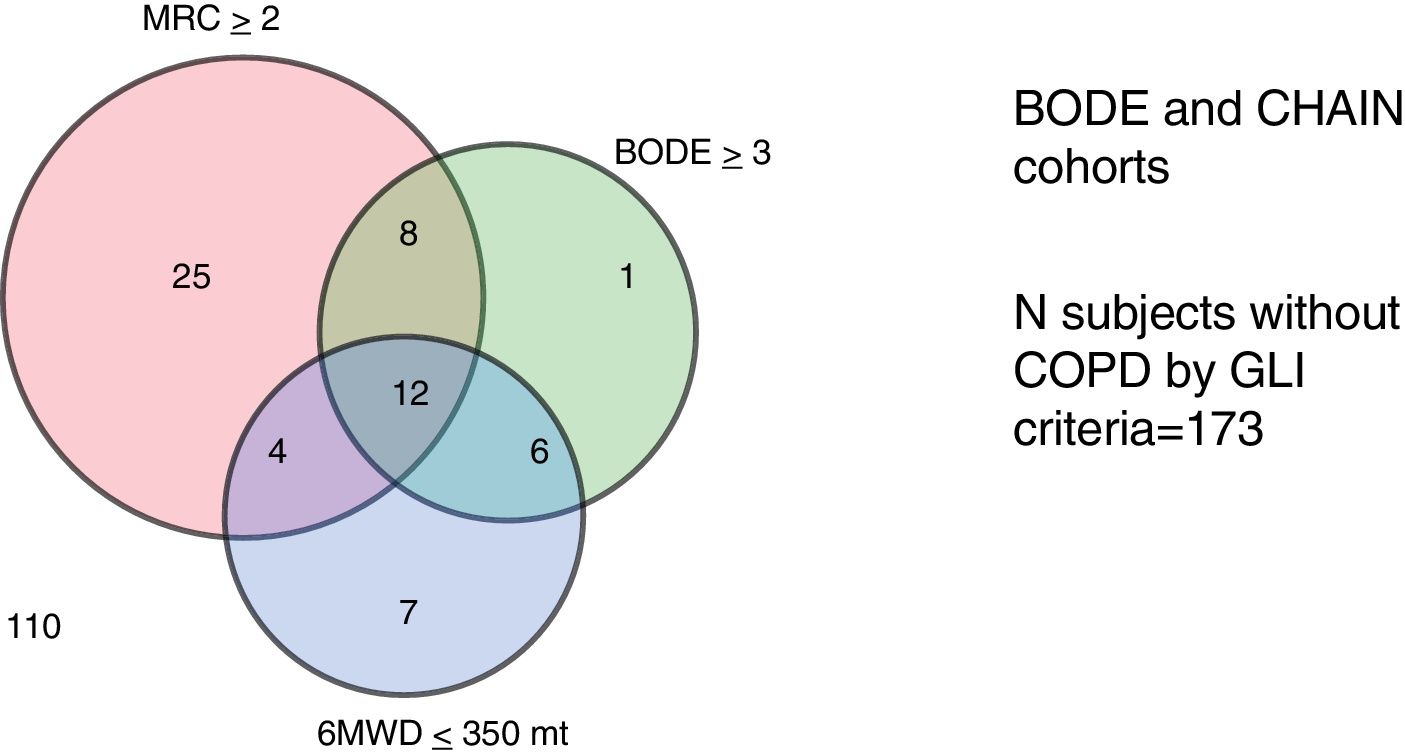
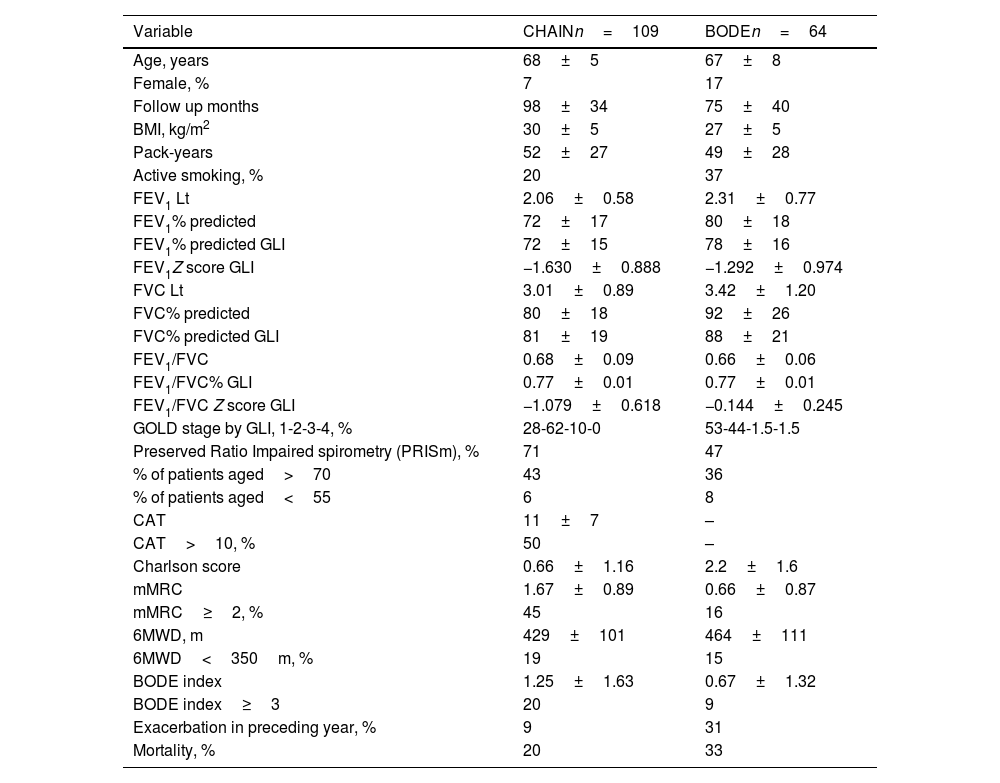
Table 2 shows the clinical and physiological characteristics of patients that did not meet GLI criteria for AL in each cohort. These patients were older, and more likely to present GOLD spirometry grades 1 and 2. No GLI AL patients were highly symptomatic, and a high proportion presented CAT>10, mMRC dyspnea≥2, 6MWD≤350m and BODE≥3. The Venn diagram in Fig. 1 shows the commonality of each of these parameters in patients with no GLI AL criteria. Interestingly, 31% of these patients in the BODE cohort and 9% in the CHAIN cohort reported a previous moderate to severe exacerbation. Long-term mortality was high among patients with no GLI AL criteria in both cohorts (20% in CHAIN and 33% in BODE). The prevalence of no GLI AL criteria was particularly high in patients>70 years old (43% in the CHAIN and 36% in the BODE cohorts). When this prevalence was explored in subjects aged<55 years, it was 6% for the CHAIN cohort and 8% for the BODE cohort.
Clinical and physiological characteristics of patients without airflow limitation based on the GLI criteria.
| Variable | CHAINn=109 | BODEn=64 |
|---|---|---|
| Age, years | 68±5 | 67±8 |
| Female, % | 7 | 17 |
| Follow up months | 98±34 | 75±40 |
| BMI, kg/m2 | 30±5 | 27±5 |
| Pack-years | 52±27 | 49±28 |
| Active smoking, % | 20 | 37 |
| FEV1 Lt | 2.06±0.58 | 2.31±0.77 |
| FEV1% predicted | 72±17 | 80±18 |
| FEV1% predicted GLI | 72±15 | 78±16 |
| FEV1Z score GLI | −1.630±0.888 | −1.292±0.974 |
| FVC Lt | 3.01±0.89 | 3.42±1.20 |
| FVC% predicted | 80±18 | 92±26 |
| FVC% predicted GLI | 81±19 | 88±21 |
| FEV1/FVC | 0.68±0.09 | 0.66±0.06 |
| FEV1/FVC% GLI | 0.77±0.01 | 0.77±0.01 |
| FEV1/FVC Z score GLI | −1.079±0.618 | −0.144±0.245 |
| GOLD stage by GLI, 1-2-3-4, % | 28-62-10-0 | 53-44-1.5-1.5 |
| Preserved Ratio Impaired spirometry (PRISm), % | 71 | 47 |
| % of patients aged>70 | 43 | 36 |
| % of patients aged<55 | 6 | 8 |
| CAT | 11±7 | – |
| CAT>10, % | 50 | – |
| Charlson score | 0.66±1.16 | 2.2±1.6 |
| mMRC | 1.67±0.89 | 0.66±0.87 |
| mMRC≥2, % | 45 | 16 |
| 6MWD, m | 429±101 | 464±111 |
| 6MWD<350m, % | 19 | 15 |
| BODE index | 1.25±1.63 | 0.67±1.32 |
| BODE index≥3 | 20 | 9 |
| Exacerbation in preceding year, % | 9 | 31 |
| Mortality, % | 20 | 33 |
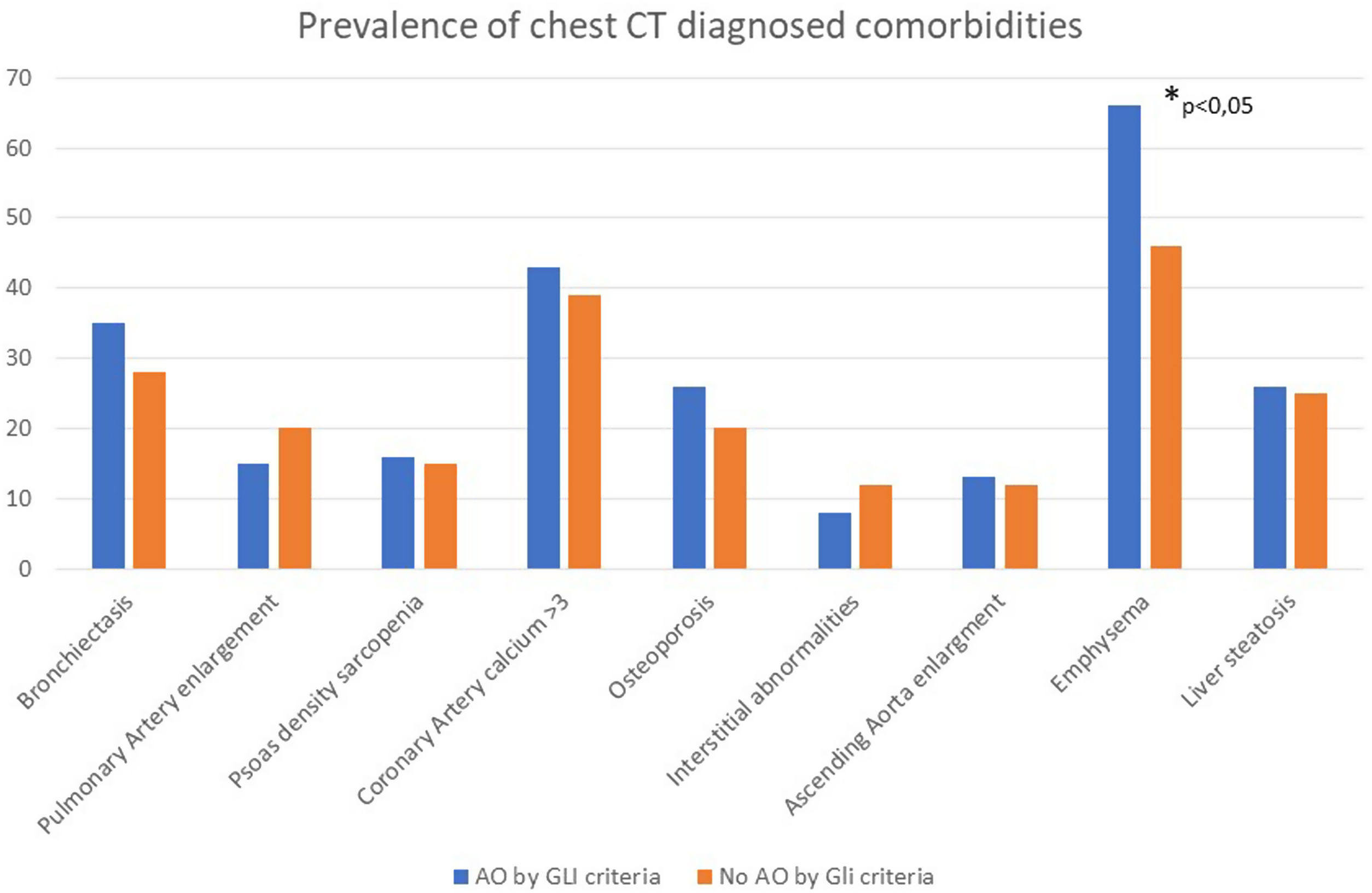
Fig. 2 shows the prevalence of CT-detected comorbidities in patients in the BODE cohort with and without GLI AL criteria. Prevalence of all CT-detected comorbidities was similar in both cohorts, except for emphysema, which was higher in patients with GLI AL criteria.
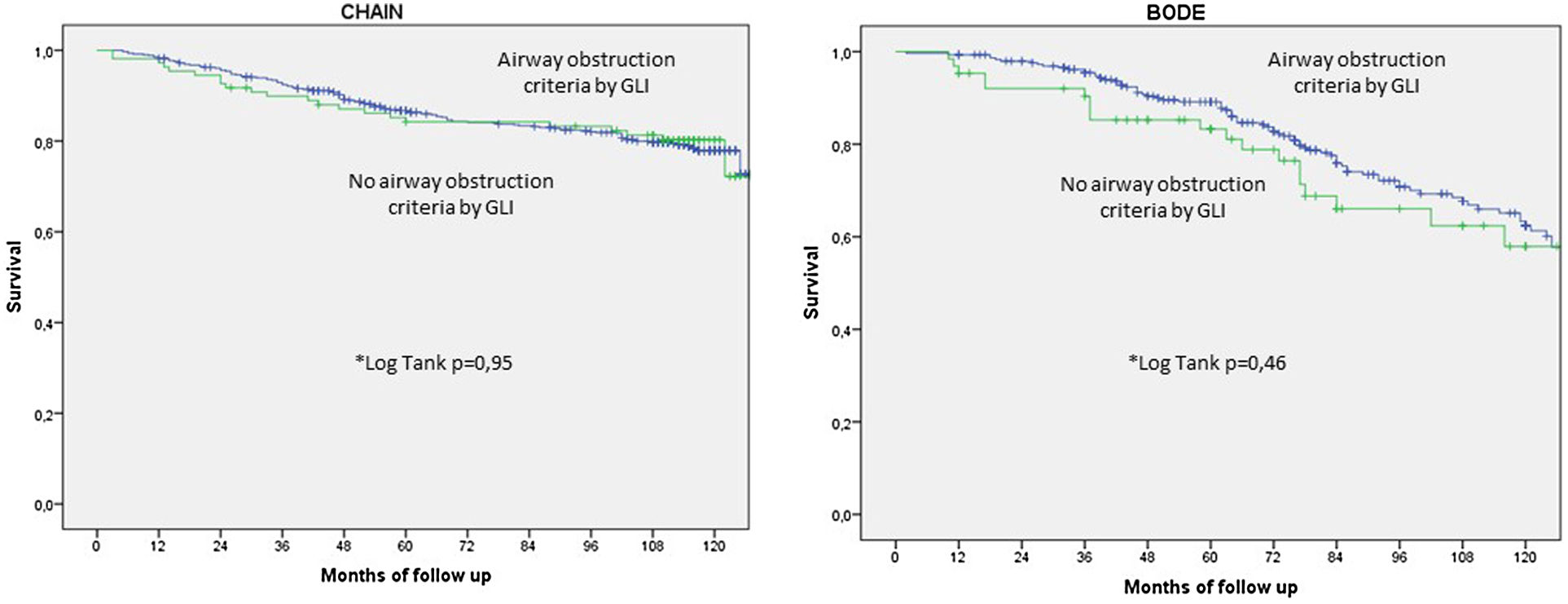
Fig. 3 shows the Kaplan–Meier survival curves for patients with and without GLI AL criteria in each cohort. Interestingly, subjects with no GLI AL criteria had similar survival as those with GLI AL criteria.
DiscussionThis retrospective analysis of 2 prospectively followed GOLD-defined COPD cohorts has shown that if the GLI criteria had been used, an important proportion of patients would not have met the threshold for airflow obstruction. However, patients with no GLI AL criteria but with GOLD COPD criteria presented considerable clinical and radiological burden of disease and mortality.
To our knowledge, this is the first study exploring the potential impact of applying the GLI airflow obstruction definition in real life cohorts of well characterized GOLD-defined COPD patients. The most important finding is that 15–18% of patients seen and followed in specialized COPD clinics did not meet the GLI AL criteria, and would therefore not have been diagnosed with COPD in clinical practice. This difference is magnified in patients aged 70 years or older, where the prevalence reached 43% in the CHAIN and 36% in the BODE cohort. It could be argued that differences in prevalence between the GOLD and the GLI criteria are due to the more “precise” methodology used in the latter, which reduces the risk of false positives in otherwise healthy individuals.3 However, the empirical evidence from the well characterized CHAIN and BODE shows that this is not the case. As shown in Table 2, 45% in CHAIN and 16% in BODE had an mMRC dyspnea scale ≥2, a score associated with increased risk of all-cause and respiratory death4,15; similarly, 19% in CHAIN and 15% in BODE achieved<350m in the 6MWT, a value that implies limited exercise capacity and poor survival over time in patients with COPD.16 The overall BODE index≥3, also associated with high all-cause and respiratory mortality,4 was observed in 20% of the CHAIN cohort and 9% of the BODE cohort.
In line with these important patient-reported outcomes, 9% of patients in CHAIN and 31% in BODE reported a moderate to severe exacerbation in the year prior to enrollment. The COPD assessment test (CAT) score was >10 in over 50% of patients. Scores higher than 10 denote a more severe impact of COPD on the patient's life.4 Importantly, as shown in Fig. 2, objective determination of prevalent comorbidities by chest CT in the BODE cohort14 was similar in patients not meeting GLI criteria for AL compared to GOLD criteria for AL, suggesting major compromise of other organ systems among patients defined as having no AL using the GLI criteria. Importantly, patients with no airflow limitation according to the GLI AL criteria presented considerable long-term all-cause mortality risk (Fig. 3). Taken together, these findings confirm that these patients were evaluated not merely on the basis of their symptoms, but also on the basis of objective evidence of respiratory involvement. The low Charlson comorbidity scores in both cohorts show that associated morbidities have no role in the clinical and mortality outcomes documented in both cohorts.
Several studies have analyzed the differences in AL using the LLN or fixed FEV1/FVC ratio to define physiological abnormality.17,18 The largest are population-based studies, and many use only pre-bronchodilator spirometric values. Other studies have suggested differences in outcomes,19–21 but provide scant information on the domains that characterize patients with COPD, such as the presence of comorbidities, CT-detected abnormalities, and importantly, long-term mortality.
Given the absence of real-world evidence studies evaluating the clinical consequences of using the GLI equations, we have had to compare our results with studies evaluating the risk of mortality in patients diagnosed using the GOLD spirometric criteria. Using this benchmark, our results are supported by large population studies. Mannino et al.5 analyzed spirometric data from over 5000 subjects enrolled in the First National Health and Nutrition Examination Survey (NHANES I), and found that all COPD groups diagnosed with FEV1/FVC ratio<0.7 had a significantly increased risk of death over the 22 years of follow-up compared with subjects with no obstruction. This includes patients classified as GOLD I, who are far less likely to meet GLI AL criteria. A similar observation was made by Lange et al.22 when they analyzed data from the Copenhagen City study of over 6000 subjects diagnosed with airflow obstruction using the GOLD criteria. Like the NHANES study, the presence of even mild airflow obstruction was associated with increased risk of death over follow-up. Recently, Batt et al.23 exploring population-based databases, confirmed that the accuracy of the FEV1/FVC<0.70 threshold for predicting COPD-related hospitalization and mortality was not significantly different from that of other fixed thresholds, but was more accurate than the LLN. Taken together, these results indicate that a mildly obstructed spirometric test (FEV1/FVC<0.7) helps select individuals who are ill, and does not over-diagnose healthy individuals. It would be fair to say that although the methodological precision with which the GLI spirometric values were calculated is admirable, the failure to measure objective variables that are useful in detecting respiratory health and outcomes in “normal” subjects undergoing testing calls into question its use in real world clinical practice.
Making a clinical diagnosis is an art that has to be based on scientific evidence. The debate about over-diagnosing COPD in “normal” individuals aged over>70 years continues to rage in the pulmonary community, with physiologists calling for more statistical precision and clinicians for practical results. This is where real world studies help clear the air. Like many other COPD studies,24,25 one third of all patients in both cohorts in our study are over 70 years of age. Thanks to major advances made in the prevention, diagnosis and treatment of COPD, life expectancy has increased significantly,26 so overlooking a significant proportion of ill patients (close to 40% in our study) in order to avoid over diagnosis of COPD is misguided, and can have a major impact on their health outcomes. It is possible – even likely – that comorbidities play an important role in the symptoms and ultimately the mortality risk of these patients,27 but the presence of significant respiratory symptoms suggests that a dysfunctional respiratory system also contributes to their overall health status, as has been demonstrated in physiological and clinical studies in patients with GOLD spirometric stage I COPD.28,29
The main strength of this study is the inclusion of 2 large, well characterized clinical COPD cohorts followed for over 7 years. This constitutes a large body of important information relating to the diagnosis and clinical course of COPD.4,16,29,30 Our study also has several limitations, the most important being the inclusion of a relatively small proportion of women, which prevents us from extrapolating our findings to this population. Second, the cohorts included a small proportion of patients aged under 55 years, so we were unable to explore the impact of the GLI AL criteria in this age group. However, the GOLD and GLI criteria are less divergent in this age range. Lastly, our findings can only be applied to patients seen in pulmonary clinics located in tertiary university hospitals. However, this is the population in which spirometric diagnosis is most widely used to select patients likely to benefit from COPD therapy. Application of the GLI criteria would have ruled out COPD in these symptomatic individuals.
In conclusion, our study shows that using the GLI AL criteria to diagnose COPD would have mis-diagnosed a significant proportion of patients with an important disease burden, and could have aggravated the existing problem of underdiagnosis of a disease that remains the third cause of death worldwide. As recently suggested by Quanjer et al.,2 caution must be taken in imposing application of the GLI criteria for AL in real world clinical practice. Perhaps the saying “Perfection is the enemy of good” can be applied to spirometric testing.
ContributionsConceptualization: JPdT, BC. Investigation: JPdT, DO, JMM, CC, CC, MM, AE, BGC, CF, IS, JGG, BC. Reporting: JPdT, DO, JMM, CC, CC, MM, AE, BC, CF, IS, AN, JGG, BGC. JPdeT is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Data sharingData related to the present manuscript can be shared upon request.
FundingThe CHAIN cohort study in Spain has been supported by an unrestricted grants from AstraZeneca and Menarini. The companies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.