Sleep apnea-hypopnea syndrome (SAHS) is a highly prevalent entity that occurs in up to 34% of men and 17% of women,1 and remains underdiagnosed in many cases. SAHS has been associated with endothelial dysfunction and atherosclerosis, processes that are also aggravated by obesity and other metabolic problems closely associated with this respiratory disorder.
Leptin is a peptide hormone produced in adipose tissue that regulates energy homeostasis and satiety in the central nervous system; leptin levels indicate the degree of adiposity,2 and there is increasing evidence of its role in the inflammatory cascade and in cardiovascular pathophysiology.3
SAHS leads to an increase in cardiovascular disease burden, and the detection of signs of early atherosclerosis in these patients could complement risk stratification. In addition, correlating leptin with ultrasound markers of atherosclerosis may provide information on the possible role of the hormone in the vascular wall thickening process.
We conducted a case-control study to investigate the relationship between serum leptin levels and Doppler ultrasound parameters in the common carotid artery in patients with SAHS. The protocol was approved by the local ethics committee and complied with the Helsinki Declaration. We included 28 individuals: 14 subjects who met criteria for moderate (apnea-hypopnea index [AHI] between 15 and 30) and severe (AHI > 30) on nocturnal polysomnography (PSG)4 (Bitmed® model NGP-340), who formed the case group, were paired with 14 other healthy subjects of similar phenotype (age, body mass index [BMI], neck circumference), who formed the control group. Blood was drawn from all participants for laboratory analysis, including the determination of fasting baseline serum leptin (Leptin Human ELISA, Clinical Range® BioVendor, Czech Republic), and a Doppler ultrasound of both common carotid arteries was performed (MayLab 30 Esaote, 7.5 MHz linear transducer) by a cardiologist experienced in the technique and unfamiliar with the arm to which the patients had been assigned and the findings of the other tests. Intima-media thickness (IMT), peak wave velocity (PWV), and intraluminal diameter of the common carotid artery, right and left, 10 mm from the bifurcation were quantified. Continuous variables are shown as means (±standard deviation) and categorical variables are shown as frequencies and percentages. Comparisons between the groups were made using the Student t test for continuous variables and the Chi-squared test for categorical variables. Multivariate logistic and linear regression models were used to evaluate the associations between PSG parameters, leptin levels, and ultrasound markers. Statistical analysis was performed using the SPSS 20.0 statistical package (IBM Corp., Armonk, NY, USA).
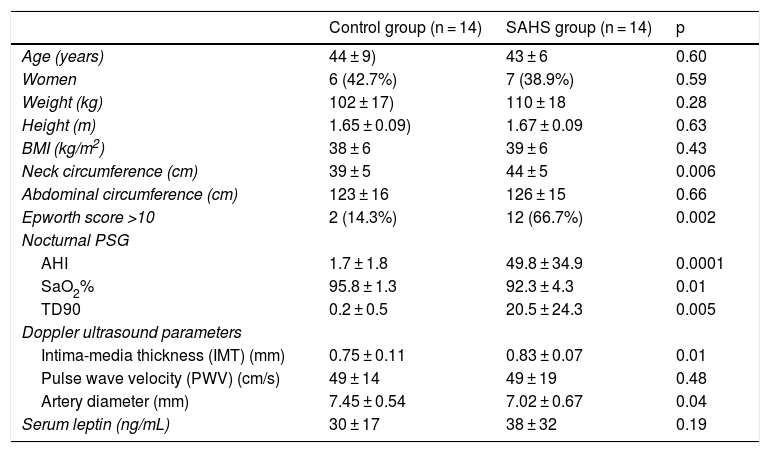
Table 1 shows the characteristics and findings of both groups. There were no differences in gender or anthropometric characteristics, except for neck circumference, which was greater in the SAHS group. Carotid IMT was larger and intraluminal diameter smaller in patients with SAHS compared to the control group (p < 0.05). There was a significant correlation between higher AHI and lower SaO2% with higher IMT and higher PWV (IAH-IMT r = 0.54, p = 0.025; IAH-PWV r = 0.5, p = 0.007; SaO2%-IMT r = −0.49, p = 0.007; SaO2%-PWV r = −0.51, p = 0.006). In terms of absolute values, leptin levels were higher in individuals with SAHS, but this difference did not reach statistical significance. Higher leptin levels were associated with lower mean nocturnal saturation (SaO2%) (r = −0.42, p = 0.02), and serum hormone concentrations correlated positively with left common carotid artery IMT in the entire series (r = 0.36, p = 0.03).
Clinical, polysomnographic, ultrasonographic, and clinical laboratory characteristics.
| Control group (n = 14) | SAHS group (n = 14) | p | |
|---|---|---|---|
| Age (years) | 44 ± 9) | 43 ± 6 | 0.60 |
| Women | 6 (42.7%) | 7 (38.9%) | 0.59 |
| Weight (kg) | 102 ± 17) | 110 ± 18 | 0.28 |
| Height (m) | 1.65 ± 0.09) | 1.67 ± 0.09 | 0.63 |
| BMI (kg/m2) | 38 ± 6 | 39 ± 6 | 0.43 |
| Neck circumference (cm) | 39 ± 5 | 44 ± 5 | 0.006 |
| Abdominal circumference (cm) | 123 ± 16 | 126 ± 15 | 0.66 |
| Epworth score >10 | 2 (14.3%) | 12 (66.7%) | 0.002 |
| Nocturnal PSG | |||
| AHI | 1.7 ± 1.8 | 49.8 ± 34.9 | 0.0001 |
| SaO2% | 95.8 ± 1.3 | 92.3 ± 4.3 | 0.01 |
| TD90 | 0.2 ± 0.5 | 20.5 ± 24.3 | 0.005 |
| Doppler ultrasound parameters | |||
| Intima-media thickness (IMT) (mm) | 0.75 ± 0.11 | 0.83 ± 0.07 | 0.01 |
| Pulse wave velocity (PWV) (cm/s) | 49 ± 14 | 49 ± 19 | 0.48 |
| Artery diameter (mm) | 7.45 ± 0.54 | 7.02 ± 0.67 | 0.04 |
| Serum leptin (ng/mL) | 30 ± 17 | 38 ± 32 | 0.19 |
AHI: apnea-hypopnea index; BMI: body mass index; SaO2%: mean nocturnal O2 saturation; SD: standard deviation.
Carotid ultrasound findings in patients with SAHS were closer to signs of incipient carotid atherosclerosis than in the control group, and while there were no differences in serum leptin levels between groups, higher levels were associated with worse SaO2% and higher IMT. The association of SAHS with subclinical atherosclerosis on ultrasound has recently been reported in a large cohort of patients younger than 68 years.2 Previous studies3,5 have also found that patients with SAHS have higher levels of leptin compared with subjects without SAHS; the difference with our results may be explained by the small size of our sample, but we cannot rule out the possible role of circadian variation in hormone levels. High leptin levels have also been associated with greater alterations in polysomnographic parameters,6 and one of the mechanisms that could mediate this phenomenon is intermittent hypoxia, which has a stimulating effect on the release of the hormone by adipose tissue.7 Leptin is associated with an increase in sympathetic activity, an increase in blood pressure and heart rate, and also participates in inflammatory activity and oxidative stress,3 processes that are involved in conjunction in the pathogenesis of atherosclerosis. These phenomena seem to mediate a greater presence of signs of subclinical atherosclerosis in patients with leptinemia. Further studies are needed to reinforce this hypothesis.
FundingThis study received partial funding from the Foundation for the Development of Science and Technology (FUNDACITE) of Venezuela, registry no. RBV20091106.
Conflict of interestsThe authors state that they have no conflict of interests.
Please cite this article as: Velásquez-Rodríguez J, Ortiz-Maraima T, Rodríguez-Viñoles MP, Bucce R, Jorquera A, Rodríguez B. Leptina sérica y marcadores ecográficos de aterosclerosis precoz en pacientes con síndrome de apneas e hipopneas del sueño. Arch Bronconeumol. 2021;57:230–231.