Pulmonary arterial hypertension (PAH) is a progressive pulmonary vasculopathy with devastating prognosis. Only three therapeutic targets (endothelin, nitric oxide, and prostacyclin) are currently available to slow down the progression of the disease. Parenteral prostacyclin analogues are recommended in patients suffering from severe PAH with high-risk profile.1 Treprostinil, a prostacyclin analogue, improves patients’ risk profile but frequently associates severe pain at infusion site. Oral selexipag is a novel orally available non-prostacyclin selective prostacyclin receptor (IP receptor) agonist,2 currently approved to treat intermediate risk PAH patients.
We recently reported the first successful switch from subcutaneous treprostinil to oral selexipag in a stable PAH patient reporting unbearable adverse events related to treprostinil subcutaneous administration.3 Six months after treprostinil withdrawal, the patient non-invasive risk profile had slightly improved and her quality of life drastically increased. At that time invasive data were lacking. We relied on non-invasive prognostic markers (WHO FC, NTproBNP and 6MWD),4 adding echocardiogram and cardiopulmonary exercise test parameters to increase accuracy.
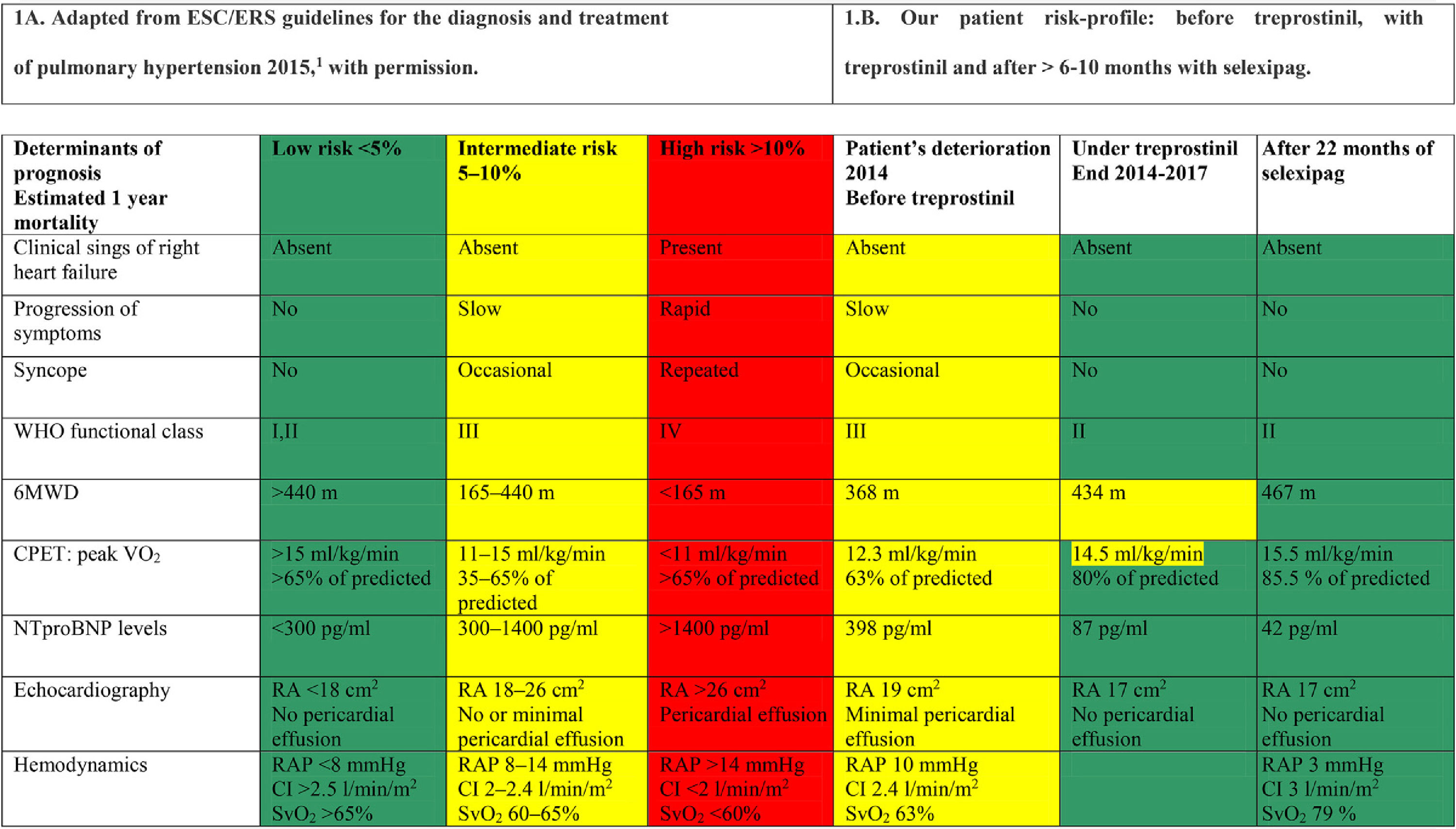
There is still little experience with transition to oral selexipag from parenteral prostacyclin therapy,5 and further investigation is required to confirm safety and efficacy of this switch. Therefore, we communicate that one year has elapsed since our patient started transition to selexipag, and treprostinil was completely withdrawn more than 10 months ago. She maintains her previously achieved low risk profile,3 and finally accepted to undergo right heart catheterization. Low risk profile achievement is now documented also invasively, and with longer follow up (Table 1).
Risk profile assessment.
6MWD: 6minutes walking distance; CPET: cardiopulmonary Exercise Test; VO2: oxygen uptake; RA: right atrium; RAP: right atrial pressure; SvO2: mixed venous oxygen saturation; CI: cardiac index.
Colors are: Red for high risk, Yellow for intermediate risk, Green for low risk. These are taking from risk evaluation table proposed in ESC/ERS Guidelines published in 2016, and used current in clinical daily practice.
Taking into account that PAH is a progressive disease, the fact that the patient remains stable, in confirmed low risk profile, 10 months after withdrawal of parenteral prostanoids, highlights the safety of transition from parenteral prostanoids to oral selexipag in adequately selected patients.