Richter syndrome (RS) consists of the transformation of chronic lymphocytic leukemia (CLL) to diffuse large B-cell lymphoma (DLBCL), a rapidly growing variety of non-Hodgkin's lymphoma with poor prognosis. The disease course in 5%–10% of patients with CLL is complicated by RS, which generally presents clinically in the form of lymphadenopathies, splenomegaly, and worsening B symptoms (fever, night sweats, weight loss).1 Positron emission tomography-computed tomography (PET–CT) is a powerful hybrid diagnostic tool that is useful in patients with RS because it helps plan and obtain histological biopsies of lesions with greater metabolism using other techniques.2,3
We report the case of a 74-year-old woman diagnosed with CLL in 2009, who attended the emergency department of our hospital with a 2-week history of progressive dyspnea, chest discomfort and low-grade fever. Chest radiograph revealed a large right pleural effusion causing mediastinal shift to the contralateral side, and secondary atelectasis of a large part of the right lung. A pleural tube was placed in the emergency room, draining abundant serosanguineous fluid. A whole-body PET–CT scan with intravenous contrast and F18-fluordeoxyglucose (FDG) performed 3 days later showed marked diffuse hypermetabolic thickening of the pleural surface of the right hemithorax, with secondary compressive atelectasis of the ipsilateral lung, which did not show pathological FDG uptake (Fig. 1). No lymphadenopathies, signs of bone marrow infiltration, or other hypermetabolic lesions were observed in the abdominal viscera or the skeleton. In view of these findings, a core needle biopsy was performed in the thickest area of the pleura with the highest metabolism on PET–CT, confirming the diagnosis of an aggressive DLBCL. The patient initially improved with pleural drainage and chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), but died 4 months later due to systemic progressive disease.
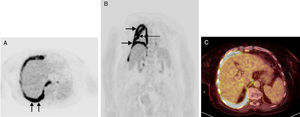
(A) PET axial image of the chest revealing hypermetabolic circumferential thickening of the pleural surface of the right hemithorax. Note the increased thickening of the posterior pleural surface (arrows). (B) PET coronal image revealing hypermetabolic thickening of the pleural surface of the right hemithorax (short arrows). Note also the intense FDG uptake by the oblique fissure (long arrow). (C) PET–CT fusion axial image showing marked hypermetabolic thickening of the pleural surface of the right hemithorax at the level of the posterior costophrenic angle. On the basis of these images, this region was selected for biopsy.
Most RS present with nodal (lymph nodes) and bone marrow involvement, although atypical forms of extranodal involvement have been described in the digestive tract, the pulmonary parenchyma and the skin. Our case is exceptional because we could not find any reports in the literature of RS presenting with exclusively pleural extranodal involvement; we only found some isolated references to RS occurring with pleural effusion, but in general, it is always associated with the presence of lymphadenopathies in other sites, with no diffuse thickening of the pleural surface. Our patient, then, is a case of DLBCL with exclusively pleural extranodal involvement complicating the course of her CLL. Two forms of primary pleural lymphoma are described in the literature: body cavity lymphoma (lymphomatous pleural effusion), which usually affects patients with acquired immunodeficiency syndrome and is caused by the human herpes virus type 8; and lymphoma associated with pyothorax, recently renamed DLBCL associated with chronic inflammation, which often affects patients with fibrothorax caused by tuberculosis, although it has also been described in association with chronic osteomyelitis of the chest wall, thoracoplasty, or the presence of metal implants (this form of primary pleural lymphoma has also been associated with Epstein–Barr virus infection).4 Our patient did not belong to either of these 2 groups of primary pleural lymphoma. In line with recent articles that highlight the importance of PET–CT in the diagnostic management of RS, performing this imaging study in our patient (as the only whole-body diagnostic imaging procedure) was very useful for demonstrating the intense hypermetabolism of the pleural surface of the right hemithorax and for ruling out hypermetabolic lesions in other sites.2,3,5 The lack of FDG uptake in the right pulmonary parenchyma, which was confirmed by the CT component of the PET–CT, prompted us to obtain a radiological-guided percutaneous core needle biopsy in the area of hypermetabolic pleural thickening.
Our case illustrates an unusual form of extranodal, exclusively pleural RS in a patient with a history of CLL. It also illustrates the great potential of the PET–CT hybrid technique in the diagnostic management of RS as a guide for obtaining histological samples of lesions with increased metabolic activity, thus increasing the diagnostic yield of the biopsy.
Please cite this article as: Gorospe Sarasúa L, Jaureguízar-Oriol A, Almonacid-Sánchez C, Rioja-Martín ME. Síndrome de Richter con extensa afectación extranodal pleural aislada: importancia de la PET/TC. Arch Bronconeumol. 2017;53:644–646.