Introduction
Diseases that constrict the skeletal structure of the chest wall and the spine with its articulations may interfere with the functional capacity of the diaphragmatic pump, facilitating the development of respiratory insufficiency and failure.
The present article discusses the response of the respiratory muscles to mechanical overload. Following a brief examination of the force these muscles are required to exert in normal conditions, we review the pathophysiology of some characteristic alterations brought about by a) chest wall diseases such as kyphoscoliosis; b) other diseases involving the bony structure of the chest wall such as ankylosing spondylitis and pectus excavatum; and c) extreme obesity.
Systemic disorders of a neuromuscular type will be dealt with in a different chapter of the series.
The structure and function of the chest wall
The chest wall is configured by the rib cage, the spinal column, and the diaphragm. The movements of the chest wall depend on a complex interaction among these components. The diaphragm forms a domelike muscular-tendinous partition which is displaced some 9.5 cm on average during tidal volume generation and which affects the chest wall by changes caused in abdominal and pleural pressures. During normal respiration, a contact point or "zone of apposition" is established between the lateral part of the base of this diaphragmatic dome and the internal surface of the lower ribs. During inspiration, the fibers of the diaphragm shorten and thus impart a caudal piston-like movement. This contraction thereby lowers pleural pressure (Ppl) and raises intra-abdominal pressure. The decrease in Ppl determines the expansion of the lungs and the increase in abdominal pressure, which is transmitted to the lower part of the chest wall via the apposition zone, which in turn makes the lower ribs expand. The movement of the ribs, which depends on their insertions into the sternum and into the vertebral column, is determined by the contraction of the parasternal muscles and the scalene muscles, which insert into the 1st to 6th ribs, and also by the costal portion of the diaphragm, which inserts into the sternum and into the 7th to 12th ribs. The muscles of the abdominal wall are important in expiration and their contraction has a significant effect on the lung volume. Electromyography (EMG) of the inspiratory muscles at rest shows that the inspiratory intercostals, the parasternals, and the scalenes act phasically in inspiration and are thus now considered accessory muscles. Deep breathing or exhausting exercise activates muscles such as the sternocleidomastoids, which should be considered the true accessory muscles.
The mechanics of inspiration and expiration
The usual end-expiratory lung volume in normal subjects (functional residual capacity or FRC) represents the perfect balance point or rest point between the elastic recoil pressures of the chest wall (PW) and the lung wall (PL). The pressure applied by the inspiratory muscles has to overcome three types of forces or resistances that oppose a change in volume: a) elastic forces, which are related to volume change; b) resistive forces, which are related to airflow (volume change per unit of time); and c) inertia, which is related to the initial force required to move tissues and air and which is not considered significant under normal conditions. The brief description presented here of the elastic properties of the lung and chest wall and of airway resistance should facilitate analysis of their complex interaction.
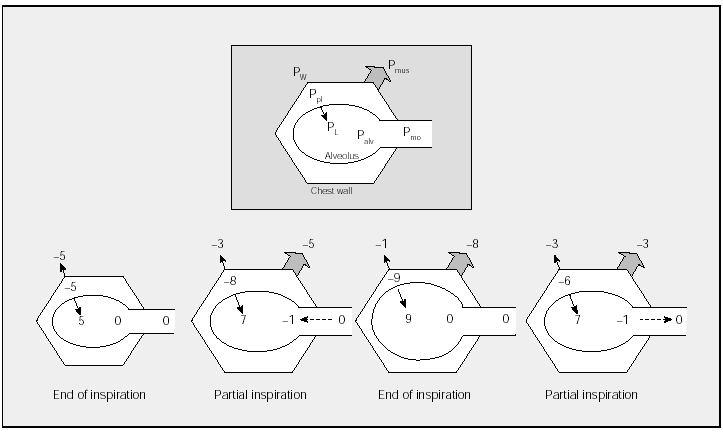
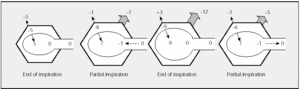
In normal conditions (Figure 1), at end expiration PL is approximately 5 cm H2O and PW is equal but opposite (-5 cm H2O). This balance produces a Ppl of -5 cm H2O and an alveolar pressure (Palv) of 0 cm H2O. Therefore, there is no airflow. The contraction of the inspiratory muscles will create an expansive force of the chest wall (Pmus) which is negative in sign and which is transmitted through the pleural space to the alveoli, where the pressure becomes subatmospheric, generating a pressure gradient and thus airflow from the mouth to the alveoli. In a partial inspiration, the negative pressure generated by the inspiratory muscles exceeds PL and a subatmospheric Palv and inspiratory airflow persist. At the end of an inspiration (at total lung capacity--TLC), a balance is established between the negative pressure exercised by the inspiratory muscles and the positive pressure exerted by PW and PL. Once again Palv is 0 cm H2O and there is no airflow. Expiration begins when contraction of the inspiratory muscles decreases; thus, the elastic recoil pressures PW and PL predominate, generating a positive Palv and expiratory flow which will continue until an equilibrium of PW and PL is reached. It is now known that there is continuous but decreasing activity of the inspiratory muscles during expiration acting as a brake on expiratory flow.
Fig. 1. Schematic representation of pressure changes in different phases of the respiratory cycle in a healthy subject. PW indicates elastic recoil pressure of the chest wall; Ppl, pleural pressure; PL, elastic recoil pressure of the lung; Palv, alveolar pressure; Pmo, mouth pressure; Pmus, muscle contraction pressure. Pressures are expressed in cm H2O. The top figure (framed) represents the equivalences; the figures of partial inspiration and expiration represent the same lung volume. See explanation in text. (Adapted from Gibson.1)
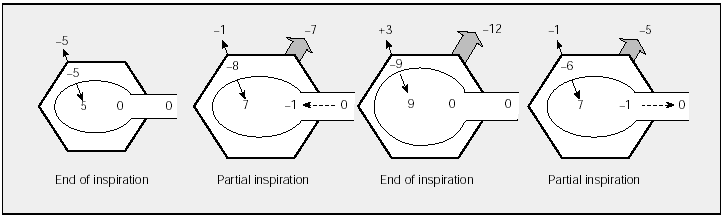
In scoliosis and other chest wall disorders, the elastic properties of the lungs and airways are normal; thus, FRC or end-expiratory pressures are normal. Since there is great reduction of chest wall compliance (chest wall stiffness) in these diseases, greater inspiratory force is required to overcome the reduction without producing very great changes in Ppl. Figure 2 shows that, assuming that chest wall compliance is half of normal, PW at the same tidal volume will increase twofold over the normal level (eg, from -5 to +3 cm H2O, as opposed to -5 to -1 cm H2O in healthy subjects) between the end of expiration and the end of inspiration. Therefore, greater inspiratory force is required to overcome chest wall stiffness.
Fig. 2. Schematic representation of pressure changes in different phases of the respiratory cycle in a subject with a chest wall disease or chest wall stiffness. See Figure 1 for equivalences. FRC indicates functional residual capacity; TLC, total lung capacity. The figures of partial inspiration and expiration represent the same lung volume. See explanation in text. (Adapted from Gibson.1)
Lung and chest wall distensibility
The lungs and the chest wall are elastic structures that tend to return to their initial shape after having been deformed by an external force. The elastic recoil pressure is that which opposes their stretch or distension. The system elasticity may be expressed as compliance and defined as volume change per unit pressure change (ΔV/ΔP).
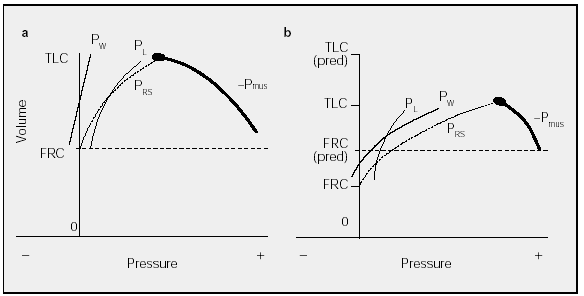
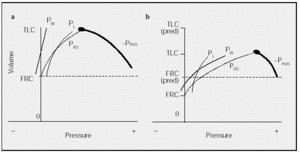
In the healthy subject a Rahn diagram (Figure 3a) illustrates that TLC is determined by the balance between the pressures generated by the respiratory muscles and the elastic recoil of the respiratory system. In a deep breath with an open glottis, Palv is zero (transrespiratory system pressure, or PRS, equals -Pmus). In patients with chest wall diseases, which are characterized by increased chest wall stiffness (Figure 3b), PW and thus PRS will increase, meaning that balance (PRS = -Pmus) will occur at a much lower volume than that observed in healthy persons, such that the pressure-volume curve is flatter. Because the volume is reduced, the maximum PL is lower than normal.
Fig. 3. Pressure-volume curve determinants in a healthy subject (a) and in a subject with chest wall disease or chest wall stiffness (b). PW indicates elastic recoil pressure of the chest wall; PRS, transrespiratory system pressure; PL, elastic recoil pressure of the lung; Pmus, muscle contraction pressure; FRC, functional residual capacity; TLC, total lung capacity. Pressures are expressed as cm H2O. See explanation in text.
Evaluation respiratory muscle functions
The mechanical properties and function of the respiratory muscles can be evaluated by measuring lung volumes, chest wall motion, and pressures at different locations of the chest wall. Respiratory muscle activity can also be characterized by EMG.
In the respiratory system, force is measured as pressures, and muscle contraction is measured as volume changes or displacement of chest wall structures. To characterize the respiratory muscles quantitatively, we can use measurements of volume, displacement, pressures, and the relationships between changes in these variables over time. Thoracic geometry has an important role to play in efficiently converting force into pressure. These pressures also depend on the mechanical characteristics of the thoracic cage and the abdominal wall, with which respiratory muscles interact. A stiff chest wall resists the distortion generated by muscle action and thus permits the diaphragm to create greater pressure for a given volume change. Therefore, the pressures should be seen only as indices of the overall work of the respiratory muscles and not as a direct measure of their contraction. The global evaluation of the respiratory muscles has been dealt with in other chapters of this series; thus, we need not enter into a discussion of this particular aspect at this time.2
Kyphoscoliosis
Kyphoscoliosis is a disease of the vertebral column and its articulations, characterized by the simultaneous presence of scoliosis (lateral curvature of the spine) and kyphosis (anteroposterior spinal curvature). Signs of the latter may be destruction of vertebral bodies, bone tuberculosis, osteoporosis, etc. Primary or idiopathic kyphoscoliosis is a hereditary disease characterized by defects in the development of the vertebrae or by connective tissue disease. It accounts for 80% of cases and is more frequent in women. Secondary kyphoscoliosis generally originates in childhood neuromuscular diseases such as poliomyelitis or muscular dystrophies.3
During the course of kyphoscoliosis, the initial displacement of the spine ("primary curvature") involves two structural changes: on the one hand, a secondary curvature that develops to counterbalance the primary curve and, on the other hand, a rotation of the column on its longitudinal axis, displacing the ribs of the convex side in a posterior and outward direction to form a "hump" shape while the ribs on the concave side are constricted. The intensity of the deformity can be quantified by measuring the angles of the primary convexity. With angles greater than 100º, the deformity is considered severe and the risk of respiratory failure, alveolar hypoventilation, and cor pulmonale is very high.4
Kyphoscoliosis is expressed in functional changes with decreases in TLC, vital capacity (VC), and FRC but without changes in residual volume (RV).5,6 The severity of compression is directly related to the degree of kyphoscoliotic angulation.7-9 Diminished expiratory flows are due to the restrictive process per se, not to intrinsic airway disease.10
In advanced kyphoscoliosis, inspiratory muscle strength is significantly reduced and maximum transdiaphragmatic (Pdimax) and inspiratory (PImax) pressures are much reduced.6 In such patients, high positive gastric pressures are generated during respiration, indicating that diaphragm contraction is effective with very negative esophageal pressures. This suggests active recruitment of the intercostal muscles and the inspiratory accessory muscles. In studies of kyphoscoliosis patients, an inverse correlation has been observed between PaCO2 and PImax,, possibly indicating that inspiratory muscle restriction plays a predominant role in the development of respiratory failure in these patients.
In patients with chronic obstructive pulmonary disease (COPD) and other similar respiratory diseases, increased nerve activity has been observed in the inspiratory muscles of the rib cage and the diaphragm. Such observations suggest that a typical response to mechanical overload is involved.11 Consistent with this hypothesis, Estenne et al12 observed a PImax, a sharp decrease in dynamic lung compliance (0.04±0.03 L/cm H2O) and an increase in respiratory muscle recruitment during respiration in 8 kyphoscoliotic patients with a severe restrictive pattern (TLC 44%±8% of predicted, FRC 60%±11% of predicted, and RV 82%±20% of predicted). The observations were attributed to an automatic response of central respiratory drive.
In summary, decreased respiratory system compliance, conditioned by the stiffness which deformity transmits to the chest wall,13 is evident in adults with kyphoscoliosis. This brings about a reduction in the TLC and flattens the pressure-volume curve (Figure 3b). The respiratory load increases so that patients adopt a breathing pattern characterized by small volumes and very high respiratory rates,14 with increased dead space, alveolar hypoventilation, and disordered sleep,15 these changes being very marked at times. Similarly, the greater work of the respiratory muscles stimulates increased oxygen consumption, involving greater risk of respiratory failure due to muscle fatigue.16
Compliance in children, on the other hand, is preserved regardless of the severity of the deformity.17
Ankylosing spondylitis
Ankylosing spondylitis is an inflammatory disease of the axial skeleton that mainly affects males. Ninety-five per cent of affected individuals share the genetic marker HLA-B27. Inflammation compromises the ligamentous structures of the spinal column, the sacroiliac articulations, and the ribs, leading to the fibrosis and ossification of these structures and, thus, to an increase in rig cage stiffness due to ankylosis and fusion of the costovertebral and sternoclavicular articulations.
In spite of this rib cage stiffness, there is no very marked decrease in the lung volumes, either TLC or FRC,18 nor is there an observed correlation between the severity of the spinal angulation and the functional parameters indicating restriction. The slight reduction in lung volumes, which is possibly the result of the fusing of the ribs,19 correlates positively with disease duration and activity.20 Finally, observed decreases in both PImax and maximum expiratory pressure indicate weakness caused by intercostal muscle atrophy plus dysfunction of the diaphragm, whose activity is generally compensatory and preserved.
Pectus excavatum
Pectus excavatum is the most common deformity of the anterior thorax. Consisting of a very notable concavity of the lower third of the sternum, pectus excavatum is a congenital defect which is manifested in different degrees of severity. Thus, it is classified according to the extent of concavity visible in a lateral x-ray of the thorax. It is considered significant when the distance between the surface of the anterior wall of the thorax and the deepest part of the depression is greater than 3 cm.21 Its etiology is unknown, although a close relation to the Marfan syndrome has been found to exist, suggesting that the connective tissue around the sternum is altered. Lung volumes are at the lower limits of normal or slightly diminished, and compliance is normal unless accompanied by severe kyphoscoliosis.22
Obesity
When obesity is severe (a body mass index [BMI] greater than 30 kg/m²), lung function may be compromised. The distribution of obesity can also endanger respiratory function, independently of the total percentage of body fat.23 However, forced spirometry in patients with uncomplicated obesity is generally normal, although there may be a small decrease in forced VC, which correlates inversely with weight.24 The effects of uncomplicated obesity are generally reflected more in a significant decrease of the expiratory reserve volume, which equals FRCRV at <=60% of predicted. This decrease has an inverse correlation with BMI. The TLC and FRC tend to be normal25 with BMI values under 45 kg/m<². Patients with obesity hypoventilation syndrome (OHS) usually manifest more severe restrictive and functional impairment, which makes it impossible to explain the adverse effects of obesity on lung function exclusively in terms of the adipose load on the chest wall.26
In patients with uncomplicated obesity, chest and respiratory system compliance is slightly diminished (approximately 80% of predicted), reaching losses of approximately 40% when OHS is present. Decreased distensibility is due to the mechanical restriction of the thorax and to increased abdominal content and fat, which place a greater elastic load and require a greater negative Ppl during inspiration.27,28 Obesity has also been related to increased airway resistance. This can be explained in part because at low lung volumes, with increased elastic tension, there is a decrease in the diameter of the small airways (a normal ratio of forced expiratory volume in 1 second to forced VC) with a consequent increase in resistance,29 a characteristic that is most pronounced in the presence of OHS.
This functional situation requires more work to overcome the elastic and resistive forces and thus leads to greater oxygen consumption by the respiratory muscles. Kress et al30 found that oxygen consumption by respiratory muscles was greater in morbidly obese patients than in normal subjects, although it decreased with mechanical ventilation. In theory, increased loading could either have a training effect or could cause fatigue if the burden of concurrent disease became very great. In uncomplicated obesity, inspiratory muscle strength is normal.
Conclusion
Diseases that constrict the chest wall lead to severe respiratory function impairment. Kyphoscoliosis, congenital disorders of the chest wall, and morbid obesity are the chest wall disorders that most commonly lead to the development of cor pulmonale and chronic respiratory insufficiency. In patients with these disorders, a stiff thorax imposes a very considerable elastic load on the respiratory muscles. This increases the work of breathing and alters lung function mechanics. If inspiratory muscle weakness is also present, the consequences may be even greater.
Even though the pathophysiology of the respiratory muscles in such disorders has not been studied to the same extent as in COPD, there is still sufficient information on functional impairment and the role it plays in the development of respiratory failure when disease progresses or when an additional burden arises from acute (eg, respiratory infection) or chronic (eg, concurrent COPD) diseases. A more detailed study of chest wall disorders will, in many cases, make it possible to modify or prevent the natural history of a disease.
Correspondence to: Dr D. Maldonado.
Fundación Neumológica Colombiana.
Calle 163.ª, 28-60. Bogotá. Colombia.
E-mail: dmaldonado@cable.net.co
Manuscript received March 24, 2003.
Accepted for publication April 2, 2003.