Introduction
Lung transplantation is a therapeutic option indicated in patients with terminal pulmonary disease who have received all types of medical and surgical treatment, but whose condition, nevertheless, remains serious. Advances in systems for procuring and preserving the organ, immunosuppression, surgical techniques and intra- and postoperative handling, among other factors, have improved life expectancy and quality for these patients. Despite all our experience, however, perioperative mortality in lung transplantation remains high to this day.
The International Society for Heart and Lung Transplantation (ISHLT) Registry is the source that best reflects the current state of lung transplantation in the world.1 The mortality rate published for all lung transplantations (pediatric and adult, single- and double-lung) is 25% the first year, with the highest incidence (20%) in the first month after transplantation. The most important known causes of death in this first month, according to the ISHLT Registry, seem to be primary graft failure and infection.
The objective of this study was to look at our experience with the early postoperative period following lung transplantation and, by analyzing the frequency and causes of mortality in the postoperative recovery unit, to try to identify the possible pre-, intra- and postoperative risk factors associated with mortality.
Patients and methods
We analyzed retrospectively the records of 68 consecutive patients who had received a single- or double-lung transplant in the Clinica Puerta de Hierro in Madrid over a period of 56 months. Prior to this, we had excluded from the study 5 patients who had undergone lung retransplantation, a procedure involving technical difficulties which increase morbidity and mortality, for which reason they were not really representative of the program.
Ideal lung donors are considered to be those who are under 60 years of age, with a normal chest x-ray, absence of purulent secretions or bronchial aspiration, with no history of malignancy, systemic disease or significant smoking, and with a ratio of PaO2 to the fraction of inspired oxygen (PaO2/FiO2) greater than 250.2 The lung was preserved with sodium heparin (3 mg/kg), prostaglandin E1 (1 mg) and 4 L of Euro-Collins solution at 4ºC through the pulmonary artery (anterograde preservation) and the left atrial appendage (retrograde preservation), leaving the lungs inflated to 50% of their vital capacity with an FiO2 of 1. Finally the lungs were submerged in a bath of modified Euro-Collins solution at 4ºC for transport.3
The donor variables analyzed were demographic and anthropometric data (age, sex, weight and height), cause of death and intensive care unit (ICU) length of stay, and PaO2/FiO2 ratio.
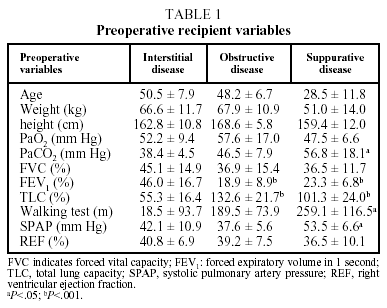
The preoperative recipient variables were demographic and anthropometric data, type of disease that led to transplantation, pretransplant lung function tests (forced vital capacity, forced expiratory volume in 1 second [FEV1] and total lung capacity), arterial blood gases, walking test and hemodynamic data (systolic pulmonary artery pressure and right ventricular ejection fraction).
The intraoperative variables we included were the type of transplantation carried out (single- or double-lung), whether or not extracorporeal circulation (ECC) was used and graft ischemic time.
Finally, postoperative bleeding requiring reoperation and early graft dysfunction (EGD) in the early postoperative period were evaluated as risk factors associated with mortality. Taking the PaO2/FiO2 ratio as the basic method of evaluating graft function, we considered a PaO2/FiO2 ratio less than 150 lasting more than 4 hours during the first 24 hours following transplantation to be indicative of EGD, once the presence of atelectasis, hemothorax, pneumothorax or cardiogenic pulmonary edema had been ruled out by x-ray.
All the data obtained were analyzed with SPSS 10.0 software. We compared the variables in different groups using Student t, χ⊃, Mann-Whitney U, or Fisher exact tests, depending on the type of variable. A P value <.05 was considered significant.
A univariate analysis was done to identify the variables associated with the incidence of mortality in the postoperative recovery unit. The variables that showed statistical significance were entered into multivariate analysis, using a logistic regression model, to calculate odds ratios (OR) and 95% confidence intervals (CI). The OR was defined as the ratio of the possibilities of death in the group with a given risk factor to the possibilities of death in the group without it.
Results
Donors
The mean age of the donors was 27.5±12.4 years (range 7-57). A total of 48 donors were males (71%) and the remaining 20 females (29%). The causes of death were brain injury (62%), stroke (35%), and asphyxia (3%). The mean predonation ICU length of stay was 42.8±33.5 hours (range 12-168). The mean PaO2/FiO2 ratio was 439.9±89.1 (range 234-640).
Preoperative variables
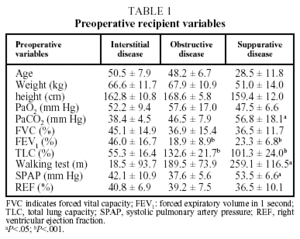
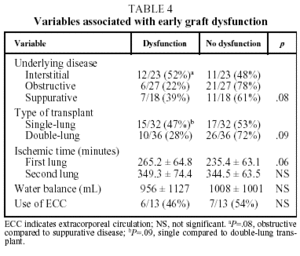
The diseases that led to transplantation were classified into 3 diagnostic groups: a) obstructive disease (n=27), including emphysema (n=8), chronic obstructive pulmonary disease (n=17), lymphangioleiomyomatosis (n=1) and bronchiolitis (n=1); b) interstitial disease (n=3), including idiopathic pulmonary fibrosis (n=20), silicosis (n=2) and sarcoidosis (n=1); and c) suppurative disease (n=18), including cystic fibrosis (n=13) and bronchiectasis (n=5).
The parameters that made up the preoperative profile of the population of recipients, including blood gas values, lung function tests, walking test and hemodynamic data are presented in Table 1. The 3 diagnostic groups showed no significant differences in patient characteristics before transplantation, although the patients with suppurative disease had a lower mean age, weight, and height than those in the other 2 groups. This group also showed greater hypoxemia and hypercapnia (P<.05) than the other 2 groups.
The FEV1 of patients with obstructive and suppurative disease was less than 25% of the expected value, significantly lower (P<.05) than that of patients with interstitial disease, while the latter had a mean total lung capacity of less than 60%, significantly lower (P<.05) than the other 2 groups.
Furthermore, the results of the preoperative walking test showed higher values (P<.05) for the group with suppurative disease. Finally, in pretransplantation echocardiographic or hemodynamic studies, this group had higher systolic pulmonary artery pressures than patients with interstitial or obstructive disease (P<.05), although no significant differences were observed in mean right ventricle ejection fraction findings.
Intraoperative variables
Thirty-two patients (47%) received single-lung transplants and 36 patients (53%) received sequential double-lung transplants.
The mean graft ischemic time for single-lung transplants was 258.1±64.8 minutes (range 120-420). In the case of double-lung transplants, the mean ischemic time was 229±56.8 minutes (range 135-350) for the first lung and 345.7±64.9 minutes (range 240-510) for the second.
The intraoperative decision to use ECC was taken on 13 occasions (19%), the most frequent reason being severe hypoxemia and/or hypercapnia prior to implantation of the first and/or the second lung. Mean ECC time was 177±105 minutes (range 28-290).
The mean duration of anesthesia and surgery was 242±95 minutes for single-lung transplants and 350±206 minutes for double-lung transplants.
Postoperative variables
There were 6 cases (9%) of reoperation due to postoperative bleeding and 25 patients (37%) showed clinical signs of EGD.
Mortality
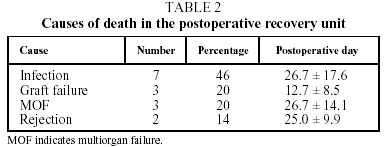
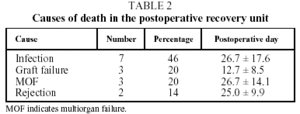
There were no cases of intraoperative mortality in the series. During their stay in the postoperative recovery unit a total of 15 patients (22%) died, which represents a survival rate with transfer to the ward of 78%. The causes of death, according to the classification used by the ISHLT Registry, were infection in 7 cases (46%), nonspecific graft failure in 3 (20%) and multiorgan failure in 3 (20%). In the 2 remaining cases (14%) the primary cause of death was attributed to rejection (Table 2).
For each of these causes the period of maximum incidence was postoperative recovery. Thus, nonspecific graft failure was the primary cause of death in the first 15 days following transplantation (P=.08), while infections were the most frequent cause of death in the postoperative recovery unit after that time and overall.
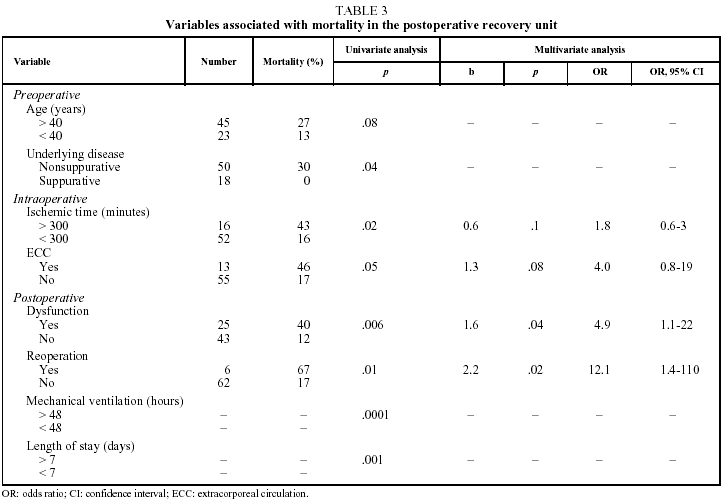
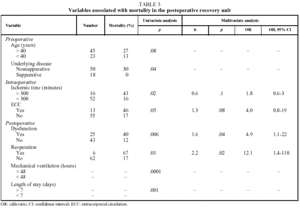
Table 3 shows the variables which, of all those studied, were associated with a higher mortality rate in the uni- and multivariate analyses.
The preoperative variables associated with mortality were age over 40 years (P=.08), underlying obstructive or interstitial disease (P<.05) and a PaCO2 value before transplantation less than 40 mm Hg (P<.05).
The intraoperative variables associated with mortality were graft ischemic time longer than 300 minutes (P<.05) and the use of ECC (P<.05).
Postoperative variables associated with mortality that remained statistically significant (P<.05) in the multivariate study were reoperation due to bleeding (P<.05) and clinical signs of EGD (P<.01).
The remaining donor-related variables (age, sex, cause of death, ICU length of stay, PaO2/FiO2 ratio) or recipient-related variables (sex, lung function tests, hemodynamic data, walking test) were not significant risk factors associated with mortality in the postoperative recovery unit. Nor did the type of transplantation performed (single- or double-lung) show significant statistical correlation with higher mortality in the early postoperative period (28% mortality [9/32] in single-lung transplants vs 17% [6/36] in double-lung transplants, [P=.2]).
Discussion
The results obtained with lung transplantation in terms of mortality are the best proof of its validity as a treatment option. In our series, the actuarial probability of survival was practically identical to the one reflected in the ISHLT Registry.4 Although this registry does not specify mortality in the postoperative recovery unit, we can take as a reference the figure of 20% mortality in the first month following transplantation. In our study, mortality in the unit was 22%, which means that 78% of our patients could be transferred to the ward in good functional status.
The final causes of death we found in our series (Table 2) were predominantly infection (46%), multiorgan failure (20%) and primary graft failure (20%), whose pathophysiological, clinical and radiological characteristics resemble those of acute respiratory distress syndrome.5 Thus, these causes of death were very similar to those of other patients with prolonged ICU stays and mechanical ventilation, whether transplanted or not.
We tried to identify the primary factors associated with unfavorable postoperative outcomes and mortality in the postoperative recovery unit.
The donor variables analyzed showed no correlation with recipient mortality, which is to say the variables included in current donor selection criteria (age, cause of death, ICU length of stay, etc) seem to have no effect on final mortality rates. However, given that the number of available donors is one of the most important limiting factors in the development of transplantation programs, many groups tend to use more liberal selection criteria, especially with respect to donor age.6 The use of such so-called "suboptimum" donors can have an effect on the final result.
We found 3 preoperative variables associated with mortality in the univariate analysis. First, there was the age of the recipient. While the influence of age on posttransplantation mortality is universally accepted, in our series we only found a tendency towards a statistically significant correlation with higher mortality (P=.08) beginning at age 40. A larger study population would probably make our results concur with those suggested by the data of the ISHLT Registry, with its enormous recipient population, which finds a greater risk of mortality in recipients over 50 (OR=1.09). The risk of mortality in patients over 60 (OR=1.33) is even more pronounced.1 The second preoperative factor associated with a significant increase in mortality was the presence of a PaCO2 value less than 40 mm Hg before transplantation, in both the univariate (P<.05) and multivariate analyses (P<.05; OR=4.4). A search of the literature yielded no support for this correlation. This can probably be explained by the zero mortality in our group of patients with suppurative disease, who were precisely those who had a significantly higher PaCO2 value than the rest. In any event, it does not appear reasonable to consider pretransplantation hypercapnia to be a protective factor against pretransplantation mortality.
The third preoperative factor associated with mortality is the previous diagnosis. In our series, the patients diagnosed with suppurative disease had 0% mortality in the immediate postoperative period, as opposed to the 30% mortality rate for the other 2 groups (P<.05). The influence of the underlying disease on posttransplantation mortality varies in different studies, although there are reports from Spanish7 and international8 groups that confirm high survival rates among patients with suppurative disease even though they present factors classically associated with higher mortality rates (greater hypoxemia and hypercapnia, pulmonary hypertension, malnutrition, bacterial colonization, etc). However, the young age of the recipient group, the better results obtained on the walking test, the aggressive antibiotic prophylaxis and the early treatment of digestive complications, among other factors, can explain the group's better results.
The intraoperative variable studies showed, in the first place, that graft ischemic time longer than 300 minutes correlated with mortality, but only in the univariate analysis (P<.05), with no confirmation in the multivariate analysis (P=.4). The lack of correlation between ischemic time and mortality was reported as long ago as 1993 by Winton et al9 of the Toronto Lung Transplantation Group, in a review of 108 single- and double-lung transplantations. In this retrospective review, they compared the incidence of postoperative complications, transplant rejection, posttransplantation functional capacity and mortality in transplants with ischemic times of more and less than 5 hours, finding no significant differences between the 2 groups.
Furthermore, Vaquero et al10 of the Hospital Reina Sofia, Cordoba, Spain transplant team, in a retrospective analysis of 45 patients, published in 1998, also failed to find any statistically significant correlation between ischemic and intubation time, duration of ICU stay or rate of ICU mortality.
Finally, no correlation between ischemic time and mortality is suggested by the ISHLT Registry data.1
All of these data serve to corroborate the impression that within reasonable limits of ischemic time (4-6 hours), quality of preservation rather than ischemic time itself is the key factor affecting graft function and postoperative morbidity and mortality. For this reason, it is logical to assume that any advance in preservation techniques that allows us to prolong ischemic time safely, without the limitations imposed by distance and time, will contribute to improving results, making both the procurement and distribution of organs a more flexible and effective process.
The use of ECC, the second intraoperative variable studied, was also significantly associated with postoperative mortality in the univariate analysis (P<.05), although this correlation was not confirmed in the multivariate analysis (P=.08) either. The data coincide, at least partially, with those of a retrospective study of 94 lung transplantations done by the University of Pittsburgh group in which the use of ECC doubled the rate of early posttransplantation mortality (14% vs 7%), without, however, demonstrating a statistically significant correlation (P=.3).11 The deleterious effects of ECC have been well documented.12 ECC sets off a systemic inflammatory response associated with fluid retention and capillary permeability and vasomotor tone disorders that alter the physiological functions of various organs, among them the lungs. Added to these effects are other complications, especially the risk of embolism and bleeding, which fully justify ECC use being considered a risk factor.13 It is interesting to observe that the patients in our series on whom ECC was used had a greater incidence of reoperation due to bleeding (23% vs 5%; P<.05) in the postoperative period. That is probably the reason why we found a higher mortality rate in our series associated with ECC (46% vs 16%; P<.05), although the multivariate analysis did not fully confirm its influence as a risk factor. Considering the size of our sample and the limitations it places on the interpretation of the results, we can conclude that extrapulmonary complications, especially bleeding, are responsible for the higher mortality rate in this group of patients, rather than ECC itself.
Reoperation due to bleeding and EGD were the noteworthy variables associated with greater transplantation mortality.
Postsurgical bleeding requiring reoperation is relatively infrequent after chest surgery, with an incidence of about 3%, although the mortality rate associated with it is as high as 23%.14 Data on the incidence and consequences of this complication in lung transplantation are scarce. In small series incidence varies between the 4% (2 cases out of 40 transplantations) reported by the transplant team of the Hospital La Fe of Valencia, Spain15 and the 9% (2 cases out of 22 transplantations) reported by the Bordeaux Lung and Heart-Lung Transplant Group.16 The St. Louis group reported the need for only 1 reoperation (1%) due to bleeding out of 102 double-lung transplants.17
In our series reoperation due to bleeding occurred in 6 cases out of a total of 68 reviewed, which represented an incidence of 9% and was associated with a postoperative mortality rate of 66% (4/6). The association between bleeding and mortality was significant, in both the univariate (P<.01) and multivariate (P<.05; OR=12.1) studies, although bleeding was not considered to be the final cause of death in any of the cases. The final cause of death was listed as infection in 3 of the 4 patients and multiorgan failure in the fourth.
The higher incidence of hemorrhagic complications we observed after lung transplantation as compared to conventional thoracotomy could be explained by various factors, such as previous chest surgery or the administration of immunosuppressants with thrombocytopenic effects. Nevertheless, it is the use of ECC during transplantation that is considered by most authors to be the greatest risk factor.18,19 In our experience, 23% of the patients who needed ECC during transplantation experienced postoperative bleeding and needed reoperation, as opposed to an incidence of less than 5% in patients having undergone transplantation without ECC (P<.05).
In 1995 Kesten et al20 reported that the use of aprotinin, a kallikrein inhibitor that decreases the release of tissue plasminogen activator during ECC, helped reduce postoperative bleeding and the need for blood products by about 50% during the postoperative period following lung transplantation. Since then, this protocol has been used by most transplant teams, including ours, although there is no conclusive evidence proving that it reduces the rate of reoperation due to bleeding in these patients.
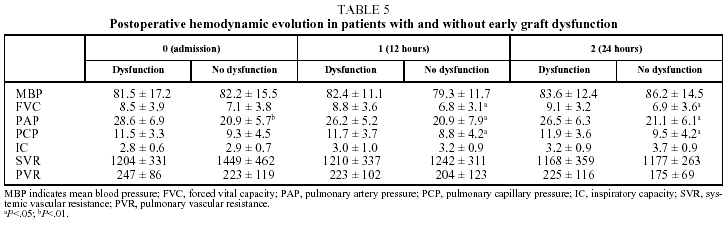
Finally, in our series, EGD, defined as severe hypoxemia (PaO2/FiO2 ratio<150) in the first 24 hours after transplantation, appeared to be closely related to mortality in both the univariate (P<.01) and multivariate (P<.05; OR=4.9) analyses. Thus, mortality among patients with EGD was 40% (10/25), compared to 12% (5/43) in the rest of the series. This is consistent with the observations of Parquin et al,21 who in a 1994 review of their experience with 92 lung transplantations reported a 20% incidence of EGD associated with an ICU mortality rate of 45%, compared to a mortality rate of 20% in patients with no signs of dysfunction (P<.05).
Later, in 1996, Plöchl et al,22 in a retrospective study of 51 lung transplantations, established that preoperative malnutrition and EGD in the early postoperative period were predictive of ICU mortality. Also in 1996, Lee et al,23 in a retrospective review of 69 single-lung transplantations, established that PaO2/FiO2 upon admission to the unit had highest positive and negative predictive value for duration of mechanical ventilation, duration of postoperative recovery unit stay, and final posttransplantation outcome than any of the other parameters studied, such as transpulmonary gradient or Acute Physiology and Chronic Health Evaluation II status. These authors identified a PaO2/FiO2 ratio less than 200 as an independent predictive indicator of posttransplantation outcome with high specificity (93%) but low sensitivity (43%).
In 1997 Boujoukos et al,24 in a new retrospective study of 30 single-lung transplantations, concluded that the presence of moderately severe radiological infiltrates were predictive of ICU stays longer than 7 days, with high specificity (80%) and sensitivity (100%). In the same study, hypoxemia, defined as a PaO2/FiO2 ratio less than 200, again showed high specificity (82%) but low sensitivity (38%) as a predictor of prolonged ICU stay.
In any event, the strong correlation between EGD and mortality in our series and others confirms our impression that, in general, most morbidity and mortality in the early postoperative period following lung transplantation is directly related to pulmonary causes, regardless of the cause of death recorded on the patient´s chart, and that any factors that might influence early graft function will therefore have a strong effect on patient outcome.
Various factors have been said to be implicated in the EGD.25 Of all of them, ischemic graft time has probably received the most attention. In 1984 Prop et al26 carried out a detailed experimental study whose purpose was to identify and analyze causes of EGD. The study included radiological tests, ventilation-perfusion scans, and histological studies carried out between the first hours and 5 months after transplantation. In that study, they established that the degree of postoperative pulmonary edema increased in proportion to graft ischemic time, corroborating the results of earlier experimental studies.27
In 1992 Kaplan et al28 confirmed these results in humans by using positron emission tomography to evaluate the degree of pulmonary vascular permeability in 15 patients during the first week after transplantation. Again, in this clinical study the pulmonary transcapillary escape rate after transplantation correlated with ischemic time.
In any event, other clinical studies have failed to support these affirmations, or at least to find a clinical relevance for them. Thus, Barroux et al29 found no correlation between ischemic time and radiological evidence of pulmonary edema during the postoperative period in 35 cardiopulmonary transplants, and we have already mentioned the results of the Hospital Reina Sofia in Cordoba, which showed no significant correlation between ischemic and mechanical ventilation time, ICU length of stay or mortality in the series.10
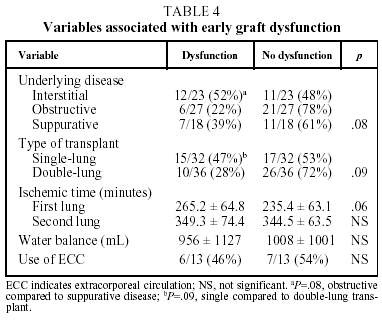
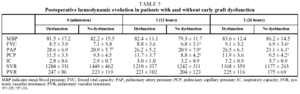
In our patients, mean ischemic time showed little statistical correlation with the appearance of signs of graft dysfunction in the postoperative period (P=.06) and although, as we have already seen, a correlation between ischemic time and mortality (P<.05) was observed in the univariate analysis, it could not be confirmed in the multivariate analysis (Tables 4 and 5).
The evidence relating ischemic time to an increase in pulmonary capillary permeability and its clinical manifestations in the form of radiological evidence of interstitial edema, hypoxemia, prolonged mechanical ventilation time or mortality is less of a contradiction than it seems if we consider that improving preservation methods can substantially reduce the undeniable deleterious effect of ischemia. Thus, reducing ischemic time does not always prevent lung damage and may be associated with severe graft dysfunction related to improper preservation.
Conclusions
The mortality rate during the early postoperative period following lung transplantation in our series was 22%.
Patients with suppurative lung disease (cystic fibrosis and bronchiectasis), despite greater preoperative hypoxemia and hypercapnia as well as higher pulmonary artery pressure, had a better survival rate than patients with obstructive or interstitial lung disease.
Postoperative bleeding requiring reoperation was a factor strongly associated with posttransplantation mortality. The incidence of bleeding was significantly related to the use of ECC and, as a result, probably increased mortality in the series.
The development of EGD is associated with longer mechanical ventilation time, a longer stay in the postoperative recovery unit and mortality, but no risk factor definitively predictive of the appearanceGD can be established.
Correspondence: Dr. F.J. Gómez Nieto.
Servicio de Anestesiología y Reanimación.
Hospital Universitario Clínica Puerta de Hierro.
San Martín de Porres, 4. 28035 Madrid. Spain.
E-mail: fjgnieto@wanadoo.es
Manuscript received November 21, 2002.
Accepted for publication February 18, 2003.