This present paper describes the general characteristics, objectives and organizational aspects of the respiratory disease registries in Spain with the aim to report their activities and increase their diffusion.
The document compiles information on the following registries: the Spanish Registry of Patients with Alpha-1 Antitrypsin Deficiency, Spanish Registry of Bronchiectasis, International Registry of Thromboembolic Disease, Spanish Registry of Occupational Diseases, Spanish Registry of Pulmonary Artery Hypertension, Registry of Pleural Mesothelioma, Spanish Registry of Tuberculosis and Spanish Multi-center Study of Neuroendocrine Pulmonary Tumors.
Our paper provides information on each of the registries cited.
Each registry has compiled specific clinical information providing data in real situations, and completes the results obtained from clinical assays. Said information has been published both in national as well as international publications and has led to the creation of various guidelines. Therefore, the activities of the professionals involved in the registries have spread the knowledge about the diseases studied, promoting the exchange of information among workgroups.
En el presente trabajo se describen las características generales, objetivos y aspectos organizativos de los registros de enfermedades respiratorias existentes en España con el objetivo de dar a conocer su actividad e incrementar su difusión.
Se recoge información sobre los siguientes registros: Registro Español de Pacientes con Déficit de Alfa-1 antitripsina, Registro Español de Bronquiectasias, Registro Internacional de Enfermedad Tromboembólica, Registro Español de Enfermedades de Origen Laboral, Registro Español de Hipertensión Arterial Pulmonar, Registro de Mesotilioma Pleural, Registro Español de Tuberculosis y Estudio multicéntrico Español de Tumores Pulmonares Neuroendocrinos.
Nuestro trabajo aporta información de cada uno de los citados registros.
Cada registro ha recogido información clínica específica que aporta datos en situaciones reales, y completa los resultados obtenidos de los ensayos clínicos. Dicha información se ha difundido en publicaciones tanto nacionales como internacionales y ha permitido la elaboración de varias normativas. Por tanto, las actividades llevadas a cabo por los profesionales vinculados a los registros han conseguido difundir el conocimiento sobre las enfermedades estudiadas propiciando el intercambio de información entre grupos.
In epidemiology, the term register is used to designate the data file concerning all the cases of a particular disease or other health conditions of defined population in such a way that the cases can be related with the base population. Such defined registers are considered population-based, while other registries are called “clinical” or “hospital” when they are restricted to the setting of one or various hospitals or patient-care systems. The population-based registers contain the information from all those centers in which patients are diagnosed and/or treated with a certain disease. They have the advantage of an available population denominator, which enables disease incidence calculations. If there is also a follow-up of the cases, then the prevalence and/or survival can also be calculated. Hospital registers refer to the experience of the institution itself, and the information is limited to the cases attended there, and their usefulness is basically clinical. Furthermore, in health-care centers there also may be clinical databases related with a disease based on the interest of a health-care professional, but these do not constitute hospital registers.
The Spanish Agency for the Evaluation of Health-Care Technologies, in compiling its directory of Health-Care Registries, defined register in the following way: a file of systematic data that is continuous and efficiently recoverable, related to elements that are important for health in a defined population in such a way that the elements registered can be extrapolated to a base population.
From this definition, one can deduce the multiple applications of the information collected in any such register and the advantages is the accumulation of quality data currently entails for biomedical research, as well as how the individual experience of professionals with a disease is supported by technological options by sharing information in order to further our knowledge.
This present study describes the general characteristics, objectives and organizational aspects of the respiratory disease registries in Spain that are mainly, although not exclusively, linked to the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) with the aim to explain their functions and increase their diffusion.
Spanish Registry of Patients With Alfa-1-antitrypsin Deficiency (REDAAT)This registry was created in 1993 after the detection of the first case in the Hospital Vall d’Hebrón (Barcelona) and the evidence of the very limited experience that existed in Spain with this disease. It forms part of the COPD (chronic obstructive pulmonary disease) research area of SEPAR.
The register consisted of a file with basic demographic and clinical data of the cases detected, and its main function was to regulate access to substitutive treatment.1 The growing availability of the product due to the improvement in the Prolastin® (Talecris Biotherapeutics Gmbh) supply network and the commercialization of Trypsone® (Institut Grifols SA) made it unnecessary to manage the substitutive treatment through the register, and the aim of the register became that of scientific diffusion.
The objectives of REDAAT are to more thoroughly understand alpha-1 antitrypsin (AAT) deficiency, stimulate research, contribute to its diffusion and improve the treatment of affected persons. Therefore, the intention of the register is to maintain a level of excellence as experts in AAT in all areas (clinical experience, research, representativity in the medical community and in patients treated), to stimulate other professionals and to motivate interest in AAT deficiency.
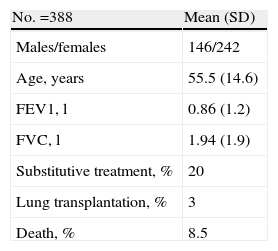
The current resources of REDAAT include: an advisory committee made up of 12 pulmonologists, 2 of whom are coordinators and one who manages the website and other diffusion activities, and 2 pediatricians. There are also those responsible for the reference laboratory and the personnel in charge of computer support. More than 200 physicians throughout Spain collaborate in the case registry. The computer application has received diverse funding: initially through an FIS grant (Fondo de Investigaciones Sanitarias – Health-Care Research Fund), later with the collaboration of Bayer Healthcare and currently with that of Instituto Grifols SL. The research projects developed have been funded by grants, both those given for projects as well as personal grants (SEPAR, Catalonian Pulmonology Society [SOCAP], FIS and international grants) and also through contributions of the aforementioned pharmaceutical companies. Recently, other additional sponsors of REDAAT activities include the companies Talecris Biotherapeutics and Crucell-Berna. The main technical resource is www.redaat.es, which legally belongs to Fundación Respira-Fundación Española de Pulmón. It includes the database of the AAT deficiency cases, that is registered in the Spanish Data Protection Agency. It has a public access area with general information and an area with restricted access for health-care professionals that includes patient data collection files as well as real-time information of the registered cases, global characteristics, diagnosis and treatment (Table 1).
The activities of the REDAAT until now could be summarized as: basic study of the disease, case detection programs,2 guidelines for diagnosis and treatment3 and information for professionals and patients.4
REDAAT has actively collaborated in the international register Alpha One International Registry (AIR, www.aatregistry.org) since its inception, and periodically exports to the international database encrypted patient data, which is registered anonymously. The board of the AIR has a Spanish representative.5
REDAAT has improved the access to the diagnosis of AAT deficiency in Spain, both in its usual variants as well as newly described variants. It also provides health-care professionals and patients with a setting where to consult about the disease, and its constancy over the years will provide new knowledge and understanding about the natural history of this genetic condition.
Spanish Registry of BronchiectasisBronchiectasis is an entity that has raised little interest in the medical literature,6 except when secondary to cystic fibrosis, and there are no available series with a sufficient number of patients in order to estimate its prevalence. It is the end result of different causes and diseases whose etiological diagnoses are only reached in approximately 50% of the series published, a percentage that depends on how rigorously they are studied. Given this entity's scarcity of epidemiological data, scientific evidence, clinical guidelines, and the heterogeneity of its management, in 2002 the Spanish National Registry of Bronchiectasis was created within the chronic bronchial suppuration group of the Area of Tuberculosis and Respiratory Infections (TRI) of SEPAR, with the aim to spread the understanding of this disease in our country. We are not aware of similar initiatives in other countries.
The registry is composed of a coordinator and an advisory committee of 3 pulmonologists. It has a website (www.bronquiectasias.es) where the data-entry form can be accessed.
Its objectives are, first of all, to compile an extensive series of Spanish patients in order to study the epidemiological data, causal etiologies, clinical characteristics and methods of treatment. Other objectives are: to unify the criteria for the etiologic study, follow-up and treatment in our country; to offer technical support for etiological diagnostic techniques that are not available in all centers and to promote multi-center research studies. The register includes an algorithm for diagnosis and the etiologic study. The diffusion of the information of the register was done by e-mail to all SEPAR members, specifically to members of the TRI Area. The register has been presented at sessions of the TRI Area.
The inclusion criteria are: patients with bronchiectasis of any age and etiology, diagnosed by CT or bronchography, regardless of its cause. The register consists of the voluntary communication of patient data: demographic, clinical, radiological, microbiological, lung function, etiology and treatment. The expenses of the design and maintenance of the database were initially funded by Chiron AS and later by Praxis Pharmaceutical, Grupo BioPraxis.
To date, 1925 cases have been registered from 22 centers throughout Spain.
One of the first fruits of this initiative has been the preparation of SEPAR Guidelines for bronchiectasis.7
The bronchiectasis registry has provided a system for data collection and diagnostic testing sequence that make it easier to correctly catalogue the affected patients.
International Registry of Thromboembolic Disease (RIETE)This register was founded in January 2001 and belongs to the Spanish Society of Internal Medicine (Sociedad Española de Medicina Interna - SEMI) and SEPAR. There are more than 200 centers ascribed, with one researcher per center. The participating centers are mainly from Spain, but also from France, Italy, Israel and other countries.
Initially, its main objective is to improve knowledge about thromboembolic disease (TED) in order to optimize the treatment of the patients excluded from clinical assays due to special circumstances (pregnant women, seniors, those with renal failure, history of recent hemorrhages, disseminated neoplasms, etc.).
However, by including consecutive patients, we are coming to better understand other aspects of the disease, such as diagnostic strategies, the prognostic value of the different variables and long-term evolution.
The database includes more than 370 variables, including clinical, imaging, biological, treatment and follow-up. The register is sponsored in Spain by Sanofi-Aventis and internationally by Bayer HealthCare Schering Pharma.
Its initial line of work is the treatment of the thrombosis and pulmonary emboli in special situations, given that one of every 4 patients with venous thrombosis or pulmonary embolism has at least one exclusion criterion for participating in anti-thrombotic treatment clinical assays.
We are not aware of the existence of similar initiatives in other countries, but as non-Spanish centers participate in the register, its character is multi-centered and international.
Currently, this register has led to 56 publications in the literature. Their results have allowed researchers to make relevant progress in the understanding of TED aspects in the most complex patients and in the evaluation of prognostic and evolutional factors that would not have been possible without such a large cohort.8–12
Spanish Registry of Occupational Diseases (EROL)In 1998, an initial pilot registry was constituted to deal with occupational respiratory diseases in Catalonia, similar to the Surveillance Work Occupational Respiratory Diseases (SWORD) registry in the United Kingdom (National Heart and Lung Institute, Brompton Hospital, London). In 2002, it became effective in 3 areas of Spain (Asturias, Catalonia and Navarra).
Several societies participate in this register: the Spanish Society for Clinical Immunology and Allergies (Sociedad Española de Inmunología Clínica y Alergia -SEICAP), the Spanish Society for Work Safety and Medicine (Sociedad Española de Medicina y Seguridad del Trabajo – SEMST) and SEPAR. In the meetings and congresses of all these scientific societies, the results are usually presented, while attempts are made at stimulating the participation of more regions. Furthermore, on the web pages of some of these societies, such as that of SEPAR, there are data available from the registry that all members may consult.
Currently, the coordinators of the 3 registers mentioned are members of SEPAR. In Asturias, the Asturian Institute for the Prevention of Occupational Risks (IAPRL), and in Navarra, the Institute of Navarra of Occupational Health (INSL), promote, finance and oversee the registries in those areas.
In Spain, these registers of occupational diseases have fundamentally been centered on mandatory notification in cases of incapacity due to work-related disease. The most important objective was to evaluate the existence of under-notification of occupational respiratory diseases, as was happening in other countries, in addition to providing data about frequency, causes and determinants (place/time), risk tendencies and differences between participating areas. On the other hand, the registry could alert other professionals, establish primary prevention methods and evaluate methods of control for certain incidences.
In Asturias and Navarra, notifications are made by standard mail. In Catalonia, notifications are generally made through a website (www.malaltiaocupacionalrespiratoria.net), where the personal and general data of the cases introduced can be consulted. On occasion, there has been collaboration of administrative staff, but the work is usually done by the coordinator, occasionally helped by the notifiers.
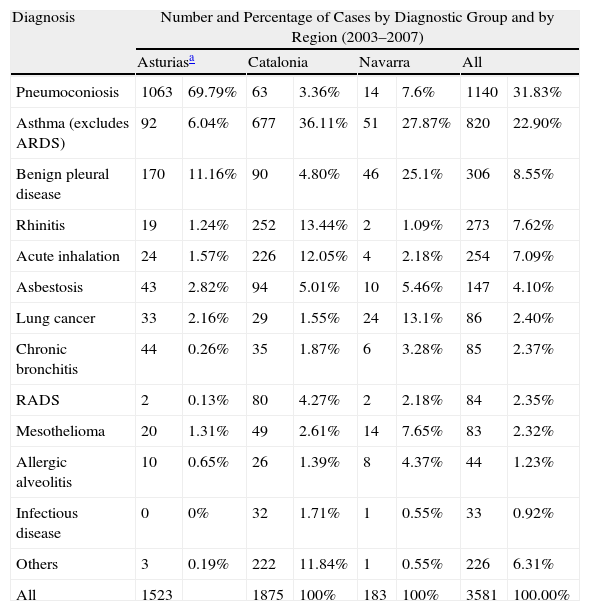
Each participating area has its own database. A summary of the cases obtained over the last 5 years (2003–2007) are shown in Table 2. The results obtained show that: the official register of the occupational respiratory diseases in Spain is very deficient; the register presented, based on voluntary notification, improves the information on the incidence and characteristics of these diseases and detects some specific particularities and considerable differences among the 3 areas studied.13–15
Number and Percentage of Cases by Diagnostic Group and by Region in the EROL Register.
| Diagnosis | Number and Percentage of Cases by Diagnostic Group and by Region (2003–2007) | |||||||
| Asturiasa | Catalonia | Navarra | All | |||||
| Pneumoconiosis | 1063 | 69.79% | 63 | 3.36% | 14 | 7.6% | 1140 | 31.83% |
| Asthma (excludes ARDS) | 92 | 6.04% | 677 | 36.11% | 51 | 27.87% | 820 | 22.90% |
| Benign pleural disease | 170 | 11.16% | 90 | 4.80% | 46 | 25.1% | 306 | 8.55% |
| Rhinitis | 19 | 1.24% | 252 | 13.44% | 2 | 1.09% | 273 | 7.62% |
| Acute inhalation | 24 | 1.57% | 226 | 12.05% | 4 | 2.18% | 254 | 7.09% |
| Asbestosis | 43 | 2.82% | 94 | 5.01% | 10 | 5.46% | 147 | 4.10% |
| Lung cancer | 33 | 2.16% | 29 | 1.55% | 24 | 13.1% | 86 | 2.40% |
| Chronic bronchitis | 44 | 0.26% | 35 | 1.87% | 6 | 3.28% | 85 | 2.37% |
| RADS | 2 | 0.13% | 80 | 4.27% | 2 | 2.18% | 84 | 2.35% |
| Mesothelioma | 20 | 1.31% | 49 | 2.61% | 14 | 7.65% | 83 | 2.32% |
| Allergic alveolitis | 10 | 0.65% | 26 | 1.39% | 8 | 4.37% | 44 | 1.23% |
| Infectious disease | 0 | 0% | 32 | 1.71% | 1 | 0.55% | 33 | 0.92% |
| Others | 3 | 0.19% | 222 | 11.84% | 1 | 0.55% | 226 | 6.31% |
| All | 1523 | 1875 | 100% | 183 | 100% | 3581 | 100.00% | |
ARDS: adult respiratory distress syndrome.
A SEPAR group grant was given to the 3 autonomous communities to help fund the initiation of the project. In Asturias, resources have been obtained from the Asturian Department of Health (Consejería de Sanidad de Asturias). In Catalonia, the funding comes from grants and aid sponsored by Mutua Asepeyo and the Catalonian Pulmonology Foundation (Fundació Catalana de Pneumologia – FUCAP). In Navarra, a grant was obtained from the Navarran Institute of Occupational Health (Instituto Navarro de Salud Laboral – INSL) in collaboration with the Universidad Pública de Navarra.
The registry is working on increasing the involvement of the health authorities and the participation of other geographical areas in the project. In 2010, it was initiated in Castilla-La Mancha.
In other countries, there are similar registers. The first was SWORD in the United Kingdom, and others have been similarly established. In the United States, the Sentinel Event Notification System for Occupational Risks (SENSOR) has made important advances in the preventive policies established in some states.
Spanish Registry of Pulmonary Artery Hypertension (REHAP)The Spanish Registry of Pulmonary Artery Hypertension (Registro Español de Hipertensión Arterial Pulmonar – REHAP) was established in 2004 within the Circulation Areas of the Spanish Cardiology and Pulmonology Societies (SEC and SEPAR, respectively), due to the need to determine the epidemiology in our country of a disease considered a rare disease, with high morbidity and mortality and a fatal short-term prognosis if left untreated. It is open to other societies (Internal Medicine, Rheumatology, and Intensive Care). Since its inception, Schering España has offered its unconditional support to the project. This company, now merged as Bayer HealthCare Schering Pharma, continues to make REHAP meetings and contacts possible with its support.
The organizational structure of REHAP is made up of 2 coordinators and an executive committee. The main objectives are: to create a data source in order to obtain an approximate situation of the disease in our country; to know the incidence and prevalence of pulmonary arterial hypertension in Spain; to homogenize the diagnostic criteria; to know the treatments used in our setting; to enable future joint studies that lead to advances in treatment; and, to facilitate the exchange of information between specialists.
Since 2007, the coordinating center of REHAP is S&H Medical Science Service. It is responsible for all the activities for collecting and managing the data of the patients included. The confidentiality of the patient, physician and hospital data is protected before being sent by means of the assignation of a unique study number for each patient at the time of the inclusion, and by means of the suppression or codification of any other information that could identify persons or hospital centers. The electronic confidential data are also protected by passwords and all the copies of the data and paper reports are stored in a safe place. The quality of the data is monitored and documented.
An expert consultant in epidemiology and statistics takes care of the periodical turnover and filtering of the data for their analysis.
The participating centers include all the patients that meet the pre-defined eligibility criteria. An essential pre-requisite is a hemodynamic study confirming the diagnosis. The inclusion of the data is the responsibility of one or two representatives per center through a safe domain (www.rehap.org). Patients are included with both idiopathic as well as associated pulmonary arterial hypertension and thromboembolic pulmonary hypertension.
The main variables compiled in the register include details of the clinical characteristics of the patients and the type, dose, duration and evolution of the specific treatment administered for the pulmonary hypertension. The endpoints of the study are transplantation or patient death.
All funding for the entire registry comes from Bayer HealthCare Schering Pharma.
The use of the data collected in the REHAP is scientific and for research use. Its infrastructure poses the possibility of group research, albeit in the fields of diagnosis, prevention or treatment of these patients, such as obtaining data for ulterior publications. Along these lines, there are guidelines regarding such publications that ensure the intellectual property of the compiled data, signing members, etc., when data from the registry are used.
There are several registers in France, Switzerland, Scotland, China, the United States, etc., but the one that deserves the greatest acknowledgement is a European register, the French Registry to be exact, which was published in 2006.
The collection of a representative sample of the individuals with PAH has allowed us to present several communications at National Congresses of both SEPAR and SEC as well as several publications.16–18 Last year, it was possible to exhibit an advance of the data about pulmonary arterial hypertension in Spain for incidence (3.15 cases/million inhabitants/year) and prevalence (15.3 cases/million inhabitants).
Spanish Registry of Pleural MesotheliomaThe Spanish Registry of Pleural Mesothelioma was conceived by the SEPAR Area of Oncology in 2006. This register was proposed after the development of another prospective register between 2002 and 2005 that had a similar overall design and in which 16 hospitals of the Community of Madrid participated.19 In 2006, the Spanish Group of Pleural Mesothelioma (Grupo Español de Mesotelioma Pleural – GEMEP) was formed, coordinated by Dr. Villena and Dr. Pun. During that year, the register was designed, all members of SEPAR were contacted and all those interested were invited to participate. In the end, 52 hospitals joined from all the Spanish autonomous communities. The objectives of the register included compiling the epidemiological, occupational and clinical data as well as those regarding diagnostic methods, treatment used and survival of these patients in our setting.20 The inclusion of the patients was prospective, from between April 1, 2008 and March 31, 2009. The Registry is supported by the collaboration of the Spanish Society of Anatomic Pathology (Sociedad Española de Anatomía Patológica – SEAP) with a participating panel of pathologists that review the sample in order to compare diagnoses.
To date, the data of 89 patients have been remitted, although other registers are still waiting to be completed in order to analyze the data. In other countries, there are registers for this disease but they do not include a geographical distribution throughout the country, do not include the cooperation of a panel of pathologists, or are retrospective.
With the data from this Registry, it will be possible to know the incidence of pleural mesothelioma in the areas of the participating hospitals, the occupational exposure of asbestos of the patients, clinical–radiological characteristics, as well as the pleural liquid, the histological type and the staging methods used, patient stages, treatment used and the survival in our setting.
Spanish Registry of TuberculosisThe Tuberculosis Integrated Research Project (Proyecto Integrado de investigación en tuberculosis – PII TB) started in January 2006 and belongs to the Tuberculosis and Respiratory Infections (TRI) area of SEPAR.
The organizational structure is based on an Executive Committee made up of 10 members, one of which is Director of the PII TB and another is the field researcher that acts as the technical consultant.
The field research is carried out by a research physician who is in charge of the coordination of the extensive team of pulmonologists that collaborate with PII TB studies. A statistician is in charge of the data analysis and a secretary takes care of the administrative duties.
The objectives are: to facilitate tuberculosis research in Spain; to incorporate the concept of evaluation in clinical practice; to stimulate the formation in research and improve the prevention and control of tuberculosis.
There is a group of 61 collaborators from 53 hospitals and centers from all over the country who provide new cases of tuberculosis through a computer application that is accessed through the SEPAR website by means of a personal password and identifier for each of the collaborators in the study. An electronic data collection notebook is used. The information obtained contains data on sociodemographics, toxic habits, anthropometrics, clinical history, diagnostic methods, study of sensitivity, pharmacological treatment, clinical evolution, treatment compliance and results.
PII TB initially was supported by SANDOZ, but currently it is exclusively financed from the institutional aid from SEPAR and research projects.
The main lines of work are the study of the disease in specific populations such as immigrants, therapeutic compliance, pharmacological toxicity and resistances. The results obtained have been presented in different congresses and scientific meetings. Material has been developed, such as the Libro blanco de la Tuberculosis (“White Book of Tuberculosis”), SEPAR guidelines and Controlando la tuberculosis (“Controlling Tuberculosis” guidelines (areas of nursing and physiotherapy),21–23 and there are various articles in press.
This register is probably a unique TB registry as it draws on the collaborations of the participants of a scientific society (SEPAR).
Registry of Neuroendocrine Pulmonary TumorsThe Spanish Multi-center Study of Neuroendocrine Pulmonary Tumors (Estudio Multicéntrico Español de los Tumores Pulmonares Neuroendocrinos – EMETNE-SEPAR) is a cooperative Spanish group created in 1999 in the Oncology Area of SEPAR.
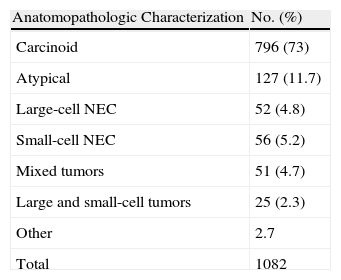
Its objective is to accumulate and compare the experience in the diagnosis and treatment of this type of tumors, especially regarding what neuroendocrine differentiation means for the prognosis. The results obtained to date and their diffusion and discussion in different forums24–27 have contributed to improving the experience in the knowledge of this disease and the results of its treatment (Table 3).
EMETNE-SEPAR studies the data of the patients affected by this type of tumor who have been surgically treated. The data, collected under conditions of absolute confidentiality and with the informed consent of the patient, are then entered in a protocol accepted by the Oncology Area of SEPAR with a register number and with no name identification, in accordance with the Spanish Law for Data Protection.
In order to enter and update cases, the researchers have a database available that contains the fields included in the protocol. Currently, 24 hospitals in Spain participate in the registry, and its members are mostly thoracic surgeons, pathologists and oncologists, in addition to renowned foreign researchers in this field. The access of the researchers to the database is done through the SEPAR website (www.separ.es), using the link that the Oncology Area has assigned the register.
Since its inception, the EMETNE-SEPAR has been given scientific and economic support by the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) and the Health-care Research Fund (Fondo de Investigación Sanitaria - FIS). EMETNE-SEPAR is inter-related with the Spanish Neuroendocrine Tumor Group (Grupo Español de Tumores Neuroendocrinos – GETNE) of the Spanish Societies of Medical Oncology and Endocrinology, and participates together with other European and American groups in managing the constitution of the International Registry of Neuroendocrine Lung Tumors.
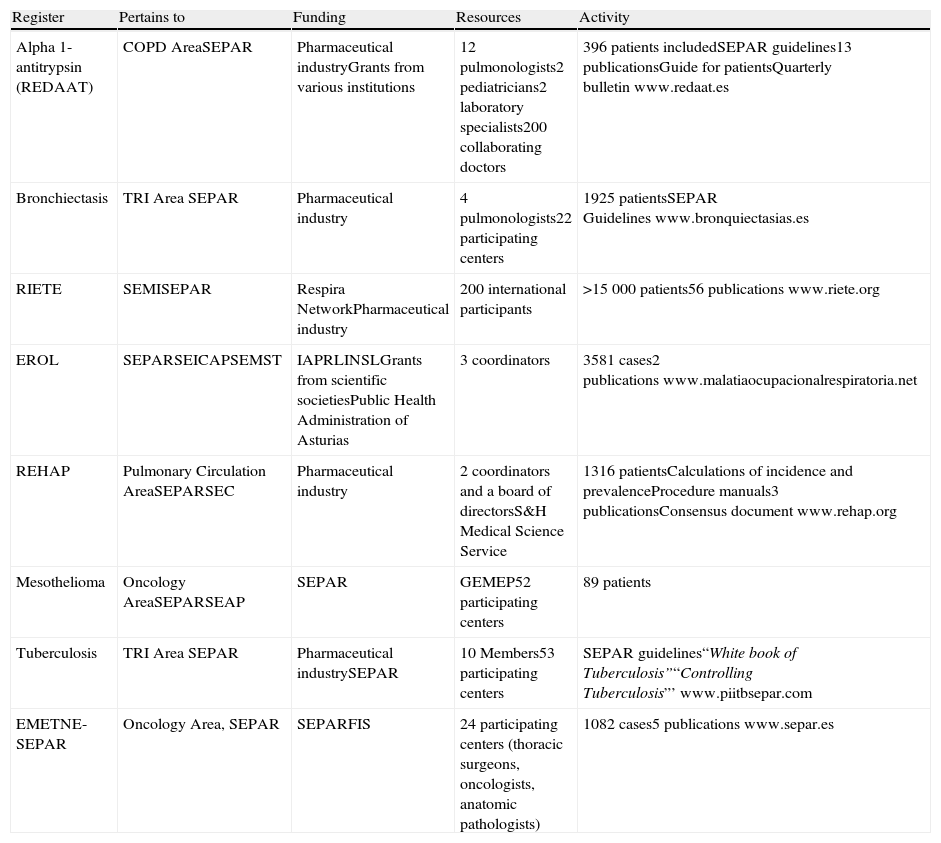
DiscussionThis present article provides information about the formation of 8 registers developed in Spain for different respiratory diseases (Table 4). These registers include rare diseases, such as AAT deficiency or PAH, and more common processes like tuberculosis. Each arose from different circumstances and also with differing resources. Nevertheless, the ultimate objective of each and every one is to improve the treatment of the affected patients through the understanding of the diseases and the evaluation of the information that is compiled. All the registries include quality information adequate for each process and have been adapted to the current legislation for data protection. In the case of rare disease registers, their value lies in the accumulation of information of the few cases in existence. In the more prevalent diseases, the value of the registers is rooted in the homogenization of criteria and the study of special circumstances that are excluded from clinical assays. For example, in the case of REDAAT, the registered population represents 3% of the individuals that are estimated to suffer severe deficiency in Spain, which reflects the situation of the important underdiagnosis that exists throughout the world. However, more than 200 physicians participate in the registry, which means that a large number of them have only one case, while the majority of pulmonologists will never diagnose a case in their entire professional career. But, if they come to do so, they have all the related information available about the situation of deficiency in Spain, including Spanish patient profiles, diagnosis, treatments, guidelines, expert panel, etc., through the registry's website.
Characteristics of the Registers.
| Register | Pertains to | Funding | Resources | Activity |
| Alpha 1-antitrypsin (REDAAT) | COPD AreaSEPAR | Pharmaceutical industryGrants from various institutions | 12 pulmonologists2 pediatricians2 laboratory specialists200 collaborating doctors | 396 patients includedSEPAR guidelines13 publicationsGuide for patientsQuarterly bulletinwww.redaat.es |
| Bronchiectasis | TRI Area SEPAR | Pharmaceutical industry | 4 pulmonologists22 participating centers | 1925 patientsSEPAR Guidelineswww.bronquiectasias.es |
| RIETE | SEMISEPAR | Respira NetworkPharmaceutical industry | 200 international participants | >15000 patients56 publicationswww.riete.org |
| EROL | SEPARSEICAPSEMST | IAPRLINSLGrants from scientific societiesPublic Health Administration of Asturias | 3 coordinators | 3581 cases2 publicationswww.malatiaocupacionalrespiratoria.net |
| REHAP | Pulmonary Circulation AreaSEPARSEC | Pharmaceutical industry | 2 coordinators and a board of directorsS&H Medical Science Service | 1316 patientsCalculations of incidence and prevalenceProcedure manuals3 publicationsConsensus documentwww.rehap.org |
| Mesothelioma | Oncology AreaSEPARSEAP | SEPAR | GEMEP52 participating centers | 89 patients |
| Tuberculosis | TRI Area SEPAR | Pharmaceutical industrySEPAR | 10 Members53 participating centers | SEPAR guidelines“White book of Tuberculosis”“Controlling Tuberculosis”’www.piitbsepar.com |
| EMETNE-SEPAR | Oncology Area, SEPAR | SEPARFIS | 24 participating centers (thoracic surgeons, oncologists, anatomic pathologists) | 1082 cases5 publicationswww.separ.es |
Abbreviations are defined in the text.
In the case of RIETE, the large number of registered cases has allowed for an evaluation of thromboembolic disease from multiple viewpoints, compiling a sufficient number of related cases with specific aspects of the disease and side effects of the treatments. This would be impossible achieve with other initiatives, even clinical assays.
The evolution of registries has reached a point that they are not merely limited to the passive storage of data. Instead, the analysis of the registers has improved our knowledge and understanding of the disease studied by means of the publication of important articles. Due to their impact in clinical practice, special mention should be given to the publication of SEPAR guidelines on the diseases studied in the registers, without which such an accomplishment would have been much more difficult, if not impossible.
All the mentioned registers are under the auspices of the main scientific societies, particularly, as they deal with respiratory diseases, SEPAR. The role of our society is determinant both in the establishment of the registers as well as in the regulation of their function and permanence. In 2005, SEPAR published regulations about the operation of the registers and has served as an interlocutor between the health-care professionals that work with them and the health system administrators when pertinent information has been requested, such as from the Spanish Ministry of Health, for instance.
Also worthy of mention is the role of the pharmaceutical industry and, in specific cases, that of other institutions, as their patronage has enabled the development of the online tools that facilitate data collection and analysis, among others.
The quality of the registries is guaranteed by their pertaining to scientific societies. These societies should continue to support such initiatives due to the great potential that these databases have in medical research, proof of which is the voluminous bibliography that has been generated. This is of particular importance in uncommon diseases, in which case it is impossible to compile ample experience on an individual basis due to their low prevalence, or to develop methodologically acceptable studies that are statistically powerful. The value of registers is becoming more and more recognized by the scientific community. Although we are accustomed to considering randomized clinical assays the gold standard for establishing the effectiveness of treatments and procedures, we must realize that they do not answer all our questions because, by definition, they are carried out under very specific conditions. Meanwhile, registries are non-interventional study tools that provide data under “real” conditions.28–30 Precisely this factor could at the same time be a limitation due to the fact that it is inevitable for there to be biases and confounding factors. As in the case of clinical assays, the quality of the data collected is essential for increasing the validity of the results extracted. Therefore, the role of the registries in treatment decision-making is still controversial, although there are some examples of how the information from a register complements or clarifies findings obtained from clinical assays.30
The survival of the registers implies increasing the confidence of both professionals and institutions in their practical usefulness and in the quality of the results that they offer. The impact of Spanish registers on respiratory diseases is not only based on the impact of the studies performed and publications but also on the involvement of Spanish professionals in similar international initiatives. The creation of registers with headquarters in our country but with participation of others promotes the establishment of global networks as well as our leadership in generating small communities of experts around each register.
In this context, more mention is being made of the relevance of quality data collection in this type of format by the most prestigious institutions and opinion leaders (WHO, Spanish Ministry of Health and Social Policy, Rare Disease Research Institute – Health Institute Carlos III), which should motivate professionals to continue along the lines of work that have been initiated, while motivating the institutions to support these initiatives with funding as well as with the implantation of general programs, such as the National Strategy for Rare Diseases, published in the autumn of 2009.
In summary, this present paper reflects the reality of the respiratory disease registries in Spain, the fundamental elements of their functions and their usefulness for the medical community.
Conflict of InterestThe authors declare having no conflict of interests.
We would like to thank the patients for their contribution towards a better understanding of their diseases. We would like to acknowledge the professionals involved for their time in registering the patient data, and also the representatives of the several pharmaceutical companies mentioned in this manuscript for their professionalism and buen hacer. Thanks also to the scientific societies for their unconditional support.
Please cite this article as: Lara B, et al. Registros de enfermedades respiratorias en España: fundamentos y organización. Arch Bronconeumol. 2011;47:389–96.