Several markers have been investigated to predict the prognosis of lung cancer. In the present study, the prognostic values of epithelial growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), E-cadherin, and p120 catenin expression were investigated by immunohistochemistry in patients with a surgically resected non-small cell lung carcinoma (NSCLC).
Patients and methodEGFR, VEGF, E-cadherin, and p120 catenin expression were prospectively determined in resected specimens from patients with NSCLC who had undergone surgery between 2003 and 2007. Patients’ and disease-related general characteristics and survival rate were recorded.
ResultsOne hundred seventeen patients with a mean age of 61.3 years were included in the study. After a mean follow-up of 27.5 months, the median survival was determined to be 44.0 months and the 5-year survival was 46.2%. The 5-year survival in negative and positive staining groups were as follows; 32% and 66.7% for EGFR (P=.02), 37.8%, and 50.7% for VEGF (P=.5), 41% and 66% for E-cadherin (P=.19), and 46% and 50% for p120 catenin (P=.27). The differentiation, N status, stage, and EGFR staining were variables significantly affecting survival (P=.001, .006, .03, and .02, respectively). In multivariate Cox analysis, the EGFR staining level and N status were variables those significantly affecting survival (P=.021 and P=.010).
ConclusionsWhile negative staining of EGFR was related with poor survival, staining of VEGF, E-cadherin, and p120 catenin were not related with survival in patients with resected NSCLC.
Para predecir el pronóstico del cáncer de pulmón se han investigado varios marcadores. En el presente estudio, mediante inmunohistoquímica se investigaron los valores pronósticos de la expresión del receptor del factor de crecimiento epitelial (EGFR), factor de crecimiento endotelial vascular (VEGF), E-cadherina y p120 catenina en pacientes con un carcinoma de pulmón no microcítico (CPNM) sometidos a resección quirúrgica.
Pacientes y métodosSe determinó prospectivamente la expresión de EGFR, VEGF, E-cadherina y p120 catenina en muestras resecadas de pacientes con CPNM que se habían sometido a cirugía entre 2003 y 2007. Se registraron las características generales de los pacientes y relacionadas con la enfermedad y la tasa de supervivencia.
ResultadosEn el estudio se incluyeron 170 pacientes con una edad media de 61,3 años. Después de un seguimiento medio de 27,5 meses, se determinó que la supervivencia mediana era de 44,0 meses y la tasa de supervivencia a 5 años era del 46,2%. En los grupos con una tinción negativa y positiva, la tasa de supervivencia a los 5 años fue la siguiente: 32 y 66,7% para la expresión de EGFR (p=0,02), 37,8 y 50,7% para la de VEGF (p=0,5), 41 y 66% para la de E-cadherina (p=0,19), 46 y 50% para la de p120 catenina (p=0,27). El grado de diferenciación del tumor, estado de N, estadio y tinción de EGFR fueron variables que afectaron significativamente a la supervivencia (p=0,001, 0,006, 0,03 y 0,02, respectivamente). En el análisis multivariante de Cox, el nivel de tinción de EGFR y el estado de N fueron las variables que afectaron significativamente a la supervivencia (p=0,021 y p=0,010).
ConclusionesAunque la tinción negativa de EGFR se relacionó con una supervivencia desfavorable, la tinción de VEGF, E-cadherina y p120 catenina no se ha relacionado con la supervivencia en pacientes con CPNM resecado.
Tumor markers are used to determine organ tumor dissemination, predict prognoses and monitor treatment more than for establishing diagnoses.1 For lung cancer, no ideal or organ-specific tumor marker has been identified.2 Currently, numerous tumor markers are being studied for the evaluation of malignant tumors, including lung cancer.
Recently, numerous target molecules have been defined that either affect the course of the malignant tumor or stop it. The epithelial growth factor receptor (EGFR) is a transmembrane glycoprotein with tyrosine kinase activity activated by the surface receptor. EGFR plays a role in motility, adhesion, cell invasion, and tumor angiogenesis.3–5 Tumor growth depends on neoangiogenesis, which, at the same time, also facilitates tumor progress and metastasis. Therefore, the magnitude of the intratumoral neoangiogenesis is related with prognosis, which is one of the controversial aspects of the research studies that are being carried out. The tumor cells can release numerous positive regulatory factors that contribute to micro-angiogenesis, among which considerable attention has been paid to vascular endothelial growth factor (VEGF).6–8
E-cadherin, a calcium-dependent cell adhesion molecule, plays a key role in the maintenance of tissue integrity. In part, the function of this molecule is mediated by the catenins.9,10
Our intention is to evaluate the under-researched roles of E-cadherin and catenin together with those of EGFR and VEGF, which have often been researched in non-small cell lung cancer (NSCLC).
In the present study in patients with NSCLC who underwent surgical resection, we determined by means of immunohistochemistry the expression of EGFR, vascular endothelial cell growth factor (VEGF), E-cadherin, and p120 catenin with the objective of revealing their effects on prognosis.
Patients and MethodsPatient SelectionIncluded for study were those patients who had undergone either lobectomy or pneumonectomy due to NSCLC at the Dr. Suat Seren Chest Diseases and Thoracic Surgery Training and Research Hospital in the Thoracic Surgery Clinic between 2003 and 2007. The anatomic pathologists prospectively evaluated all the surgical samples. Excluded from the studies were those patients who had received neoadjuvant treatment, those who underwent incomplete exeresis, those who had died within the early post-surgery period (first 3 months), those evaluated by another anatomic pathologist, those who underwent a resection of less than a lobectomy, and those who could not be followed-up. The study was approved by the research committee of the Dr. Suat Seren Chest Diseases and Thoracic Surgery Training and Research Hospital. Informed consent was obtained from each patient.
Clinical History of the PatientsAll the patients were examined in the pre-operative period with posteroanterior and lateral-projection chest radiography, complete hemogram, serum biochemistry electrocardiogram, thoracic and upper abdominal computed tomography (CT), abdominal ultrasound, and bronchoscopy. Bone gammagraphy and cranial CT were only done in patients with clinical signs or positive lab results. All the surgical material was evaluated histopathologically and classified in accordance with the extension diagnosis system of the TNM classification from 1997.11 In all patients, we carried out the systematic dissection of the lymph nodes. The post-operative histopathological evaluation followed the recommendations of the WHO.12
Patient Follow-upOne month after hospital discharge, the patients were seen every 3 months for the first 2 years, then every 6 months for another 2 years, and then at yearly intervals. At each follow-up visit, both hemogram and chest CT were done systematically. If necessary, detailed blood analyses and radiological exams were ordered. All the patients were followed-up until either the end of the study, or until their death. Lastly, the patient information was updated by evaluating the clinical histories and by contacting either them or their families. The date on which the first metastasis was detected was considered the date of the metastasis. New lesions in the same hemithorax were considered local relapses, and other lesions were considered distant metastases.
Antibody Staining Protocol for ImmunohistochemistryThe resected materials were processed in accordance with the procedures described further ahead. The biopsy samples were transferred to poly-l-lysine coated microscope slides and were incubated the entire night at 50–60°C. The slides were treated with xylene for 2×15min and 2×20min, then with 96% ethanol for 6×1.5–2min, and then were later washed 2 or 3 times with distilled water. For the recuperation of the antigen, the sections prepared for VEGF, EGFR, and E-cadherin were incubated with trypsin (Trypsin 4-Pack KIT; BioGenex, San Ramon, California, United States) at 37°C for 45min. As the enzymatic treatment was not appropriate for p120, it was warmed in a microwave oven with ethylenediaminotetraacetic acid (EDTA) (pH 8) for 20min. Afterwards, it was cooled for 15min and washed 2 or 3 times in distilled water. Each slide was dried individually. The tissue cuts were marked with a circle made with a water-proof marker (Super PAP PEN, Beckman Coulter, Marseille, France) and immediately after having been marked, drops of hydrogen peroxide were poured on. The slide was incubated for 5min and washed in water. Then, the slide was washed in a phosphate buffered saline (PBS) for 5min. After applying the solution on the slide, it was incubated for 10min, the excess was eliminated and rinsed and the slide was put in a wet flask. VEGF (A20, sc-152, 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, California, United States), EGFR (AR335-5R, 1:10; BioGenex), E-cadherin (1:10, clone 36; BioGenex), and catenin p120 (15D2, sc-23872, 1:50; Santa Cruz Biotechnology, Inc.) were left at room temperature for 1h and were used as primary antibodies. The slide was put in a liquid that had PBS, without spilling any, and afterwards it was washed, the excess eliminated and rinsed. The slide was left in PBS for 10min. The secondary antibody was transferred to the slide and left for 15min. It was washed with PBS and left for 5min. Streptavidin (Lab Vision, Fremont, California, United States) was placed on the slide and left for 15min. It was washed with PBS, which was left for 5min. After eliminating the excess and rinsing, diaminobenzidine (DAB, BioGenex) was put on the slide, which was left for 10min, and afterwards the slide was transferred to PBS. After washing in distilled water, the slide was left in hematoxylin for 1min, and immediately afterwards was washed in water. The slide was treated with 96% ethanol for 1min and then dried. After leaving it in xylene for 5min, it was covered with a cover slip. p120 catenin was only studied in 69 of 117 cases due to technical reasons that arose in the anatomic pathology department.
ImmunohistochemistryNegative staining was defined in the following way: absence of stain or stain <10% for EGFR, stain <25% for VEGF, stain <50% for E-cadherin and p120 catenin. The positive stain was accepted as values greater than these mentioned.
Statistical AnalysisThe survival rates in the different groups of patients were compared in accordance with the staining characteristics of EGFR, VEGF, p120 catenin, and E-cadherin. Due to the limited number of patients, the variables were grouped in the following way: histologic type as squamous or non-squamous; T status as T1–2 or T3–4; stage as stage I (IA and IB), stage II (IIA and IIB), or stage III (III and more); and N status as N0–1 (N0 and N1) or N2.
The statistical analyses were done with SPSS (Version 9.0; SPSS, Inc., Chicago, Illinois, United States). The comparisons between groups were carried out with the Student's t-test for parametric variables and Fisher's exact test or Pearson's χ2 test for non-parametric variables. For the survival analyses, the Kaplan–Meier method was used. The comparisons between the survival rates were carried out by means of the log-rank test using life tables and the Kaplan–Meier method. In the survival rate calculations, mortality related with lung cancer was taken into account. A multivariate analysis was done, using the forward conditional Cox model. A P value ≤.05 was considered statistically significant.
ResultsThe mean age of the 117 patients included in the study was 61.3 (range, 42–77). Table 1 presents the general patient characteristics. Adjuvant treatment was administered in 33 patients: 5 received chemotherapy, 18 radiotherapy, and 10 chemoradiotherapy. The indications of the adjuvant treatments were T3 in 3 patients, T4 in 1, N1 in 6, and N2 in 23, while 5 patients with N2 disease could not receive adjuvant treatment due to the unfavorable evaluation of their functional state. The patients were followed for a mean period of 27.5±20.0 months (range, 3–70 months). Median survival was 44.0 months and the 5-year survival rate was 46.2% at the end of the study. In 12 patients, the follow-up was more than 60 months: 6 patients survived disease-free, 5 survived relapses, and 1 died of unrelated causes.
General Patient Characteristics.
| Characteristics | n (%) |
| Sex | |
| Men | 111 (94.9) |
| Women | 6 (5.1) |
| Age, years | |
| ≤60 | 53 (45.3) |
| >60 | 64 (54.7) |
| Intervention | |
| Lobectomy | 80 (68.4) |
| Pneumonectomy | 37 (31.6) |
| Histologic grade | |
| Well-differentiated | 29 (24.8) |
| Moderately differentiated | 75 (64.1) |
| Poorly differentiated | 13 (11.1) |
| Histologic type | |
| Squamous cell | 62 (53.0) |
| Adenocarcinoma | 47 (40.2) |
| Large-cell | 7 (6.0) |
| Other | 1 (0.9) |
| T state | |
| 1 | 7 (6.0) |
| 2 | 84 (71.8) |
| 3 | 20 (17.1) |
| 4 | 6 (5.1) |
| N state | |
| 0 | 69 (59.0) |
| 1 | 20 (17.1) |
| 2 | 6 (5.1) |
| Anatomic pathology stage | |
| IA | 4 (3.4) |
| IB | 50 (42.7) |
| IIA | 3 (2.6) |
| IIB | 26 (22.2) |
| IIIA–IIIB | 34 (29.1) |
| EGFR | |
| Positive | 49 (41.9) |
| Negative | 68 (58.1) |
| VEGF | |
| Positive | 82 (70.1) |
| Negative | 35 (29.9) |
| E-cadherin | |
| Positive | 29 (24.8) |
| Negative | 88 (75.2) |
| p120 catenin | |
| Positive | 43 (62.3) |
| Negative | 26 (37.7) |
| Outcome | |
| Disease-free survival | 30 (25.6) |
| Survival with continued disease | 27 (23.1) |
| Mortality related with the disease | 51 (43.6) |
| Mortality due to other causes | 9 (7.7) |
| Relapses | |
| Present | 73 (62.4) |
| Absent | 44 (37.6) |
| Type of relapse | |
| Local | 37 (50.7) |
| Distant | 26 (35.6) |
| Local+distant | 10 (13.7) |
EGFR: epithelial growth factor receptor; and VEGF: vascular endothelial growth factor.
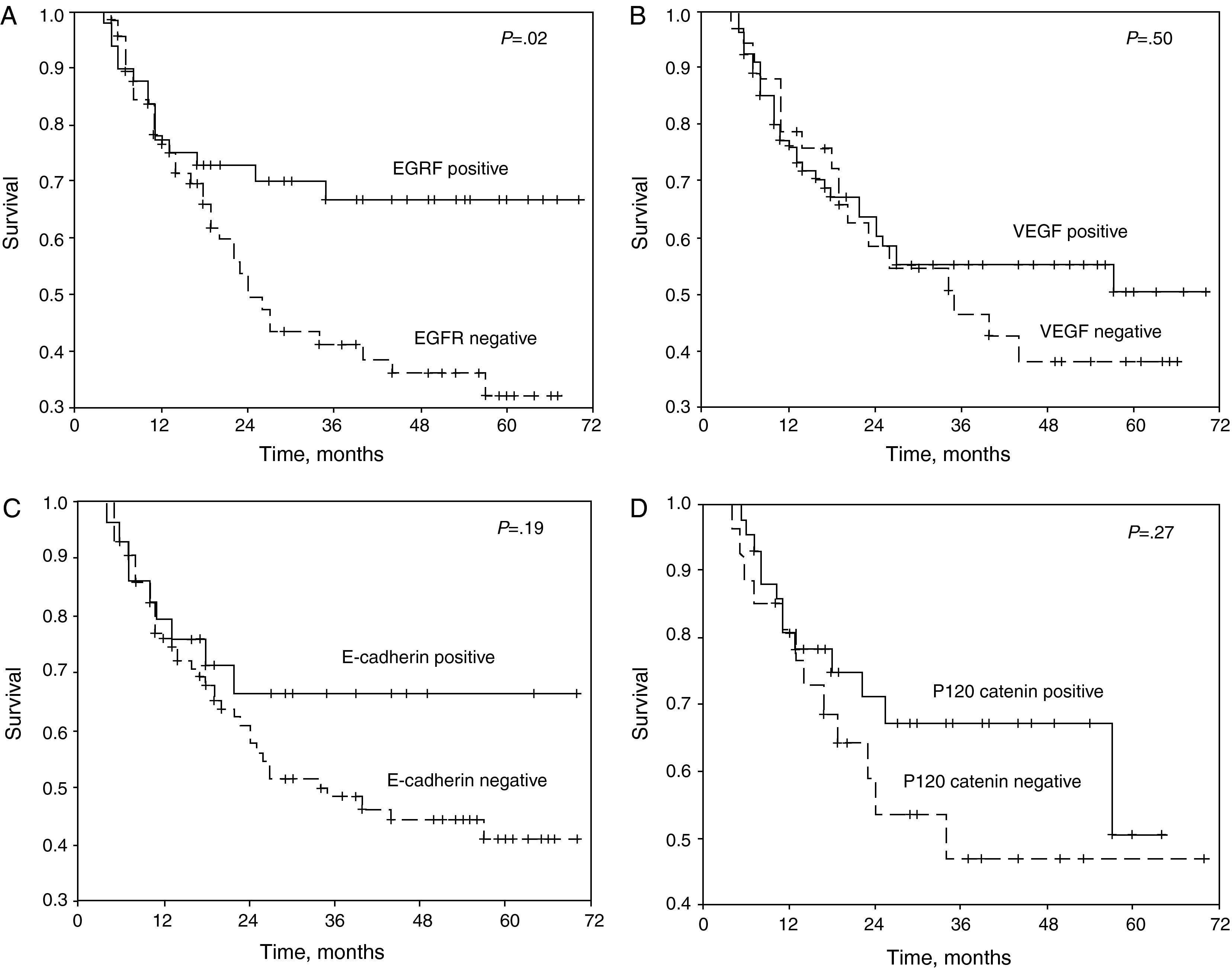
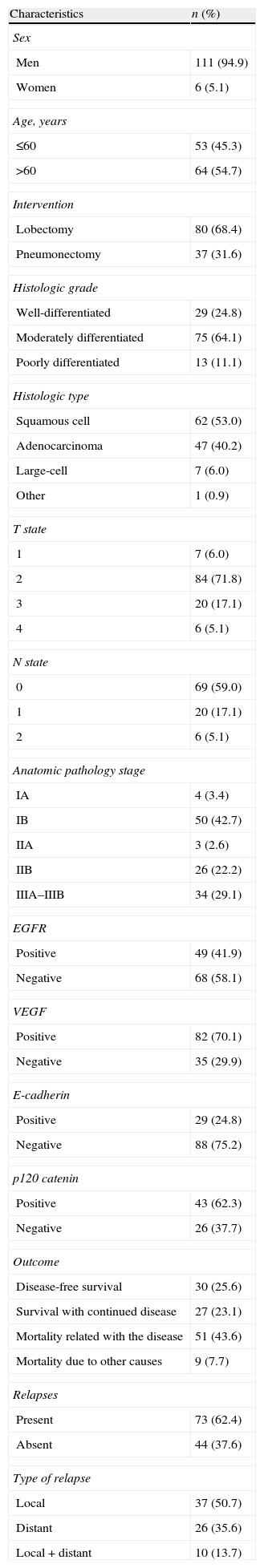
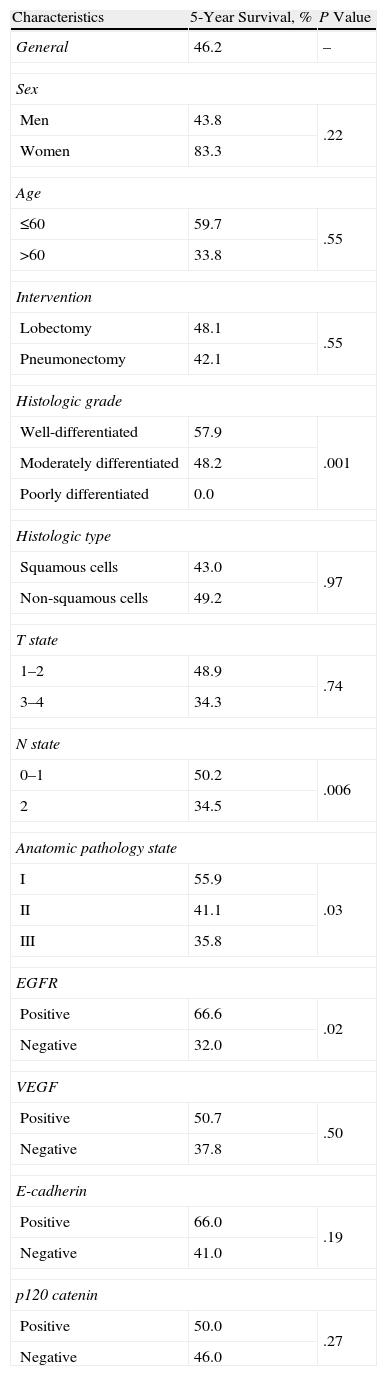
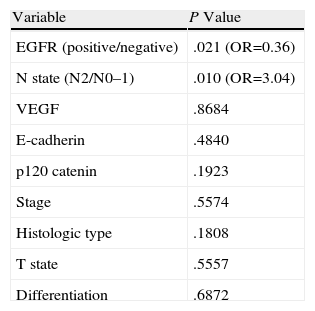
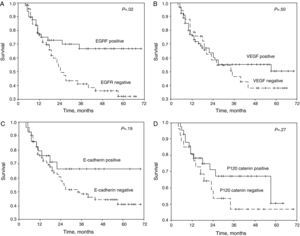
The patients who stained positive for EGFR, VEGF, E-cadherin, and p120 catenin were characterized by a greater 5-year survival (Fig. 1A–D). In the univariate analysis, the differentiation, N status, stage, and EGFR stain were variables that significantly affected survival (P=.001, .006, .03, and .02, respectively) (Table 2).
(A) Survival curve of the patients of the study according to the results of the staining for epithelial growth factor receptor (EGFR). (B) Survival curve of the patients of the study according to the results if the staining for vascular endothelial growth factor (VEGF). (C) Survival curve of the patients of the study according to the results of the staining for E-cadherin. (D) Survival curve of the patients of the study according to the results if the staining for p120 catenin.
General Survival Rates.
| Characteristics | 5-Year Survival, % | P Value |
| General | 46.2 | – |
| Sex | ||
| Men | 43.8 | .22 |
| Women | 83.3 | |
| Age | ||
| ≤60 | 59.7 | .55 |
| >60 | 33.8 | |
| Intervention | ||
| Lobectomy | 48.1 | .55 |
| Pneumonectomy | 42.1 | |
| Histologic grade | ||
| Well-differentiated | 57.9 | .001 |
| Moderately differentiated | 48.2 | |
| Poorly differentiated | 0.0 | |
| Histologic type | ||
| Squamous cells | 43.0 | .97 |
| Non-squamous cells | 49.2 | |
| T state | ||
| 1–2 | 48.9 | .74 |
| 3–4 | 34.3 | |
| N state | ||
| 0–1 | 50.2 | .006 |
| 2 | 34.5 | |
| Anatomic pathology state | ||
| I | 55.9 | .03 |
| II | 41.1 | |
| III | 35.8 | |
| EGFR | ||
| Positive | 66.6 | .02 |
| Negative | 32.0 | |
| VEGF | ||
| Positive | 50.7 | .50 |
| Negative | 37.8 | |
| E-cadherin | ||
| Positive | 66.0 | .19 |
| Negative | 41.0 | |
| p120 catenin | ||
| Positive | 50.0 | .27 |
| Negative | 46.0 | |
EGFR: epithelial growth factor receptor; and VEGF: vascular endothelial growth factor.
In addition to the values of EGFR, VEGF, E-cadherin, and p120 catenin, the Cox multivariate analysis for stage, histologic type, differentiation, and T and N status revealed that the EGFR stain level and the N status were variables that significantly affected survival (P=.021 and P=.010) (Table 3).
Cox General Multivariate Analysis.
| Variable | P Value |
| EGFR (positive/negative) | .021 (OR=0.36) |
| N state (N2/N0–1) | .010 (OR=3.04) |
| VEGF | .8684 |
| E-cadherin | .4840 |
| p120 catenin | .1923 |
| Stage | .5574 |
| Histologic type | .1808 |
| T state | .5557 |
| Differentiation | .6872 |
EGFR: epithelial growth factor receptor; OR: odds ratio; and VEGF: vascular endothelial growth factor.
Although it was demonstrated that the weak EGFR stain was related with a less prolonged survival, staining for VEGF, E-cadherin, and p120 catenin was not related with the survival of patients in whom samples were obtained from the exeresis of a NSCLC.
The overexpression of EGFR was related with the disease in advanced stage, development of a metastatic phenotype, reduced survival, and a poor prognosis.3,13–15 Although in the immunohistochemical studies of post-operative tissues it has been reported that EGFR is a negative prognostic factor in NSCLC,16 in general it is suggested that, by itself, this marker cannot be a prognostic factor.15,17,18 In the present study, in patients with negative EGFR samples, the 5-year survival rate was 32%, while in the EGFR-positive group it was 66.7% (P=.02). Although it has been suggested that the excessive secretion or the powerful staining characteristics of EGFR give rise to a predictable decrease in survival,18–20 studies have also been published documenting that it is related with longer survival, as was observed in the present study.21,22
Rusch et al.23 documented that the overexpression of EGFR was present in 70.8% of patients with NSCLC, and that these patients had a longer 5-year survival (P=.023). In a study that included a series of 408 patients with complete tumor resection, in the EGFR-positive and EGFR-negative groups the 5-year survival rates were 63% and 61%, respectively.13 In the present study, no significant differences were identified regarding the staining characteristics of EGFR and the determined stage of the disease. Likewise, nor was a significant difference detected between the positive and negative EGFR groups either for differentiation or metastases.
In some studies, it was found that the high expression of VEGF and the frequency of metastasis to the lymph nodes are related in the tumor tissue of patients with lung cancer without distant metastasis, while in others this relationship has not been reported. In patients without metastasis to the lymph nodes, no relationship has been demonstrated between tumor size and VEGF values.6,7,24 Although it has been previously suggested that there is an increase in lymph node affectation as VEGF values increase,6 in the present study this relationship was not detected. Furthermore, while in the VEGF-negative group the 5-year survival rate was 37.8%, in the positive group it was 50.7%. Despite the studies reporting that positive VEGF staining produces negative effects on survival,8,25 there are also studies available that suggest that they are related with greater survival.26,27 The results of the present study coincide with this latter group.
Sulzer et al.28 described a significant correlation between the expression of E-cadherin and greater survival. There was a significant inverse correlation between the expression of E-cadherin and the stage of the lymph nodes, just like the tumor differentiation. The reduced expression of E-cadherin correlated with the absence of histological tumor differentiation, an increase in lymphogenic metastases, and lower survival. In the present study, no significant difference was detected between the positive (24.1%) and negative (23.9%) E-cadherin groups regarding the frequency of N2. Although it was not statistically significant, in the positive E-cadherin group we observed a tendency towards a higher survival rate compared with the E-cadherin-negative patients in stage III (50% and 30.2%, respectively; P=.48).
Among the 331 patients that underwent resection, it was demonstrated that the expression of E-cadherin was preserved in 193 (58%) patients and reduced in 138 (42%) patients. Regarding this E-cadherin expression, the 5-year survival rates related with lung cancer were 66.2% in the group with preserved expression and 56.3% in the group with reduced expression (P=.065). Among the cases with reduced expression of E-cadherin as well as β-catenin, a significant, unfavorable prognosis was demonstrated compared with the cases of decreased E-cadherin or β-catenin expression and compared with the cases of preserved expression of the two.9
In a study done by Retera et al.29 in patients with resected NSCLC, the decrease in the expression of catenin was clearly related with metastasis to the lymph nodes and an unfavorable prognosis. The lower expression of E-cadherin and β-catenin were related with shorter survival.
A deficient expression of catenin is related with shorter disease-free periods and survival in patients with adenocarcinoma, NSCLC pT1–2 and pN0.30 Intense staining of β-catenin correlates with greater survival.9 In the present study, the 5-year survival rates were similar in the positive and negative groups.
The variations between the results of the study regarding survival rates are probably due to the variations in the evaluation criteria. Although the staining of a cell is considered positive for EGFR in some studies, others consider strong positive a stain >25% and weak negative a stain <25%. This causes a substantial difference in the results.
In the present study, the presence of EGFR negativity and N2 disease was related with an unfavorable prognosis. The variables that affected survival were the degree of EGFR staining and the N status, while the staining for VEGF, E-cadherin, and p120 catenin produced no effects on survival of resected non-small cell lung cancer.
Conflict of InterestsNo source of funding or support was received for the completion of this manuscript. The authors declare having no conflict of interests.
Please cite this article as: Ucvet A, et al. Valor pronóstico del receptor del factor de crecimiento epitelial, factor de crecimiento endotelial vascular, E-cadherina, y p120 catenina en el carcinoma de pulmón no microcítico resecado. Arch Bronconeumol. 2011;47:397–402.