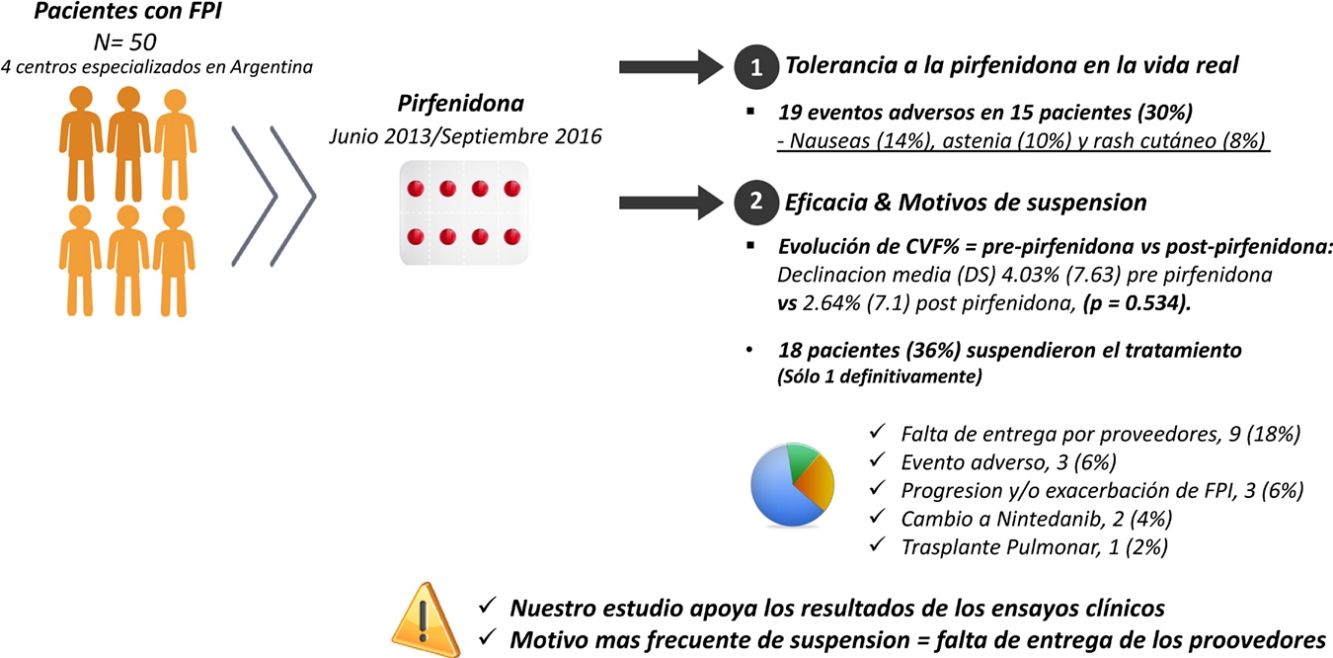
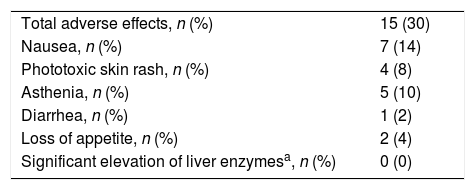Pirfenidone was the first antifibrotic drug approved in Argentina for idiopathic pulmonary fibrosis (IPF). Outcomes in real life may differ from the results of clinical trials. The primary endpoint was to study the tolerance of pirfenidone in real life. Secondary endpoints were to analyze effectiveness and reasons for discontinuation.
Materials and methodsRetrospective observational study conducted in four specialized centers in Argentina. We analyzed the medical records of patients with IPF who received pirfenidone between June 2013 and September 2016. Adverse events (AE) and the variables that could influence these results were analyzed. Forced vital capacity (FVC%) parameters were also compared between the pre-pirfenidone and post-pirfenidone periods.
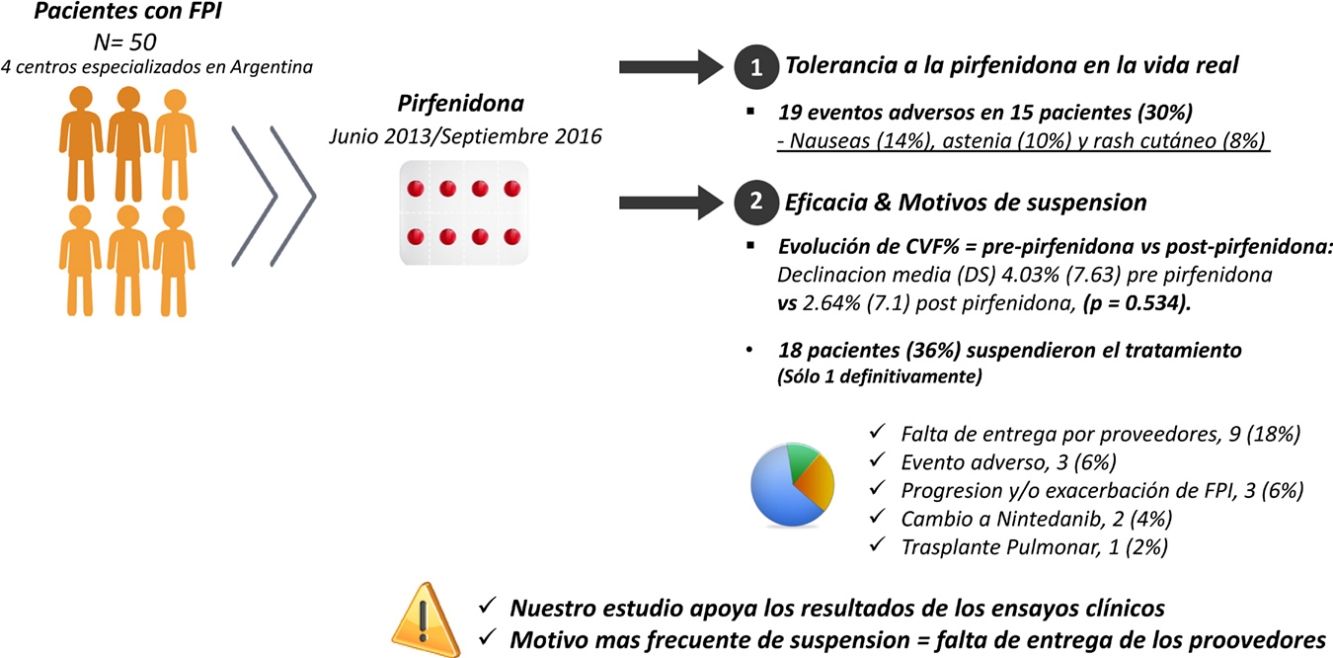
ResultsFifty patients were included, 38 (76%) men, with mean age (SD) 67.8 (8.36) years. Mean (SD) exposure to pirfenidone was 645.68 (428.19) days, with a mean daily dose (SD) of 2064.56mg (301.49). Nineteen AEs in 15 patients (30%) were reported: nausea (14%), asthenia (10%) and skin rash (8%). A total of 18 patients (36%) interrupted treatment, only 1 definitively. The most frequent reason for discontinuation was failure of suppliers to provide the drug (9 subjects; 18%). We compared the evolution of FVC% between the pre-pirfenidone and post-pirfenidone periods, and found a mean (SD) FVC% decline of 4.03% (7.63) pre-pirfenidone and 2.64% (7.1) post-pirfenidone (P=.534).
ConclusionsIn our study, pirfenidone was well tolerated and associated with a reduction in FVC decline, although without reaching statistical significance.
La pirfenidona fue el primer fármaco antifibrótico aprobado en Argentina para fibrosis pulmonar idiopática. Los resultados de los ensayos clínicos podrían ser diferentes a los de la vida real. El objetivo primario fue estudiar la tolerancia de la pirfenidona en la vida real. El objetivo secundario analizar la eficacia y los motivos de suspensión.
Materiales y métodosEstudio observacional retrospectivo realizado en 4 centros especializados de Argentina. Se analizaron las historias clínicas de pacientes con fibrosis pulmonar idiopática que recibieron pirfenidona entre junio de 2013 y setiembre de 2016. Se analizaron efectos adversos y las variables que podrían influir en la ocurrencia de los mismos. Se comparó además la evolución de capacidad vital forzada (CVF%) entre los periodos prepirfenidona y pospirfenidona.
ResultadosCincuenta pacientes, 38 (76%) hombres, edad media (DE) 67,8 (8,36) años. La media (DE) de exposición a pirfenidona fue 645,68 (428,19) días, con una dosis diaria media (DE) de 2.064,56mg (301,49). Se reportaron 19 eventos adversos en 15 pacientes (30%): náuseas (14%), astenia (10%) y rash cutáneo (8%). Dieciocho pacientes (36%) interrumpieron el tratamiento, uno definitivamente. El motivo más frecuente fue la falta de entrega de proovedores en 9 (18%). Comparamos la evolución de CVF% entre los períodos prepirfenidona y pospirfenidona, con una declinación media (DE) de CVF% de 4,03% (7,63) prepirfenidona y 2,64% (7,1) pospirfenidona, (p=0,534).
ConclusionesEn nuestro estudio la pirfenidona fue bien tolerada y ha demostrado un enlentecimiento en la declinación de la CVF, aunque sin alcanzar significación estadística.
Idiopathic pulmonary fibrosis (IPF) is a progressive, irreversible disease that leads in most cases to death within 3–5 years.1 In 2008, pirfenidone became the first specific antifibrotic drug approved for the treatment of IPF, and in 2012, it was approved in Argentina for use in patients with mild to moderate disease.2 This approval was achieved on the basis of the results from the ASCEND clinical trial, which confirmed that pirfenidone reduced decline in forced vital capacity (FVC) and improved progression-free survival and exercise tolerance.3 Although the efficacy and safety of pirfenidone was studied in 5 randomized, double-blind, placebo-controlled clinical trials, the participants in these trials met strict inclusion and exclusion criteria, and thus might not represent the heterogeneous population of patients with IPF seen in daily practice.2–6 Several real-life studies have now provided evidence on its safety and efficacy extending over a period of more than 6 years.7–9 The safety profile and effectiveness of pirfenidone may vary in different populations due to differences in genetic polymorphisms, climatic conditions, eating habits, and others. These factors underline the importance of conducting real-life studies in different countries. In Argentina, we have not yet determined the frequency of adverse events (AE) associated with pirfenidone, the impact of different clinical variables, the reasons for discontinuing treatment, and, finally, if effectiveness in real life is comparable to that reported in clinical trials. For these reasons, we decided to conduct a retrospective observational study in 4 hospitals that have multidisciplinary teams specializing in interstitial lung diseases (ILD). The main objective of this study was to determine the safety profile of pirfenidone in IPF patients in our population. The secondary objectives were to describe the reasons for discontinuation of treatment and to evaluate its effectiveness.
Materials and MethodsStudy Design and VariablesThis was a non-sponsored, retrospective, observational study, conducted in 4 hospitals in Argentina that have a specialized ILD team. We included patients with a diagnosis of IPF according to the ATS/ERS/JRS/ALAT 2011 consensus, who received pirfenidone in the period between June 2013 and September 2016.1 Patients who did not complete at least 3 months of treatment were excluded. Clinical and demographic variables included age, sex, smoking habit, the presence of symptoms of gastroesophageal reflux disease (GERD), lung pattern on high resolution computed tomography (HRCT), immunological laboratory results, need for lung biopsy for diagnosis, and development of acute disease exacerbation. HRCT pattern was categorized as typical usual interstitial pneumonia (UIP), possible UIP, and pattern inconsistent with NIU, following the recommendations of the ATS/ERS/JRS/ALAT 2011 consensus. The presence of associated emphysema was determined, and considered significant when extension was greater than 10% on HRCT. All patients were studied for rheumatoid factor (RF) (nephelometry, cut-off point 35U/ml) and antinuclear antibodies (ANA) (indirect immunofluorescence, cut-off point 1/80). Acute exacerbation was defined according to criteria proposed recently by an international study group.10 The following lung function and exercise tolerance parameters were studied: % of predicted FVC; meters walked on the 6-minute walk test; and predicted DLCO adjusted for hemoglobin. All of these variables were evaluated at the time of diagnosis, on the date of starting pirfenidone, and during treatment, according to the criteria of each medical group. Tests were conducted according to the proposals of the reference societies.11 The percentage of patients receiving treatment with proton pump inhibitors and/or N-acetylcysteine was calculated. The percentage of patients receiving systemic corticosteroids, azathioprine, and N-acetylcysteine for at least 3 months was also studied.12 To analyze the period between onset of disease and diagnosis, the number of days between the onset of symptoms (dyspnea and/or cough) and date of diagnosis in a reference center was calculated. We also analyzed the time between diagnosis and the date of starting pirfenidone. Tolerance and safety of pirfenidone were evaluated from data reported in the clinical records, such as adverse effects (AE) in the treatment period between the date of starting the drug and the date of the last visit, death or withdrawal. A significant rise in liver enzymes was defined as more than 3 times the normal value in patients who reported symptoms, or more than 5 times in those who reported no symptoms. The different clinical, functional, tomographic, and progress variables were compared between patients with and without reported AEs. To study the effectiveness of pirfenidone, the change in FVC in the period prior to receiving the drug (before pirfenidone) was compared with the change in the same variable during the period in which the patients were exposed to the drug (after starting pirfenidone). For this analysis, only patients with FVC results in both periods, with a difference of no more than 6 months between the determinations, were included. Finally, we compared the change in FVC during treatment with pirfenidone among subgroups of patients with FVC≥75% and those with FVC<75% at the time of starting treatment.
Statistical AnalysisCategorical variables were described by frequency, and continuous variables by mean and standard deviation (SD) or median and interquartile range (IQR), depending on whether distribution was normal or not, respectively. For comparative analysis, the Chi square test, Fisher's exact test, Student's test or Wilcoxon test were used as appropriate, depending on the type of variable and its distribution. A P-value of <.05 was considered statistically significant. Results were communicated according to the indications of the STROBE initiative.13
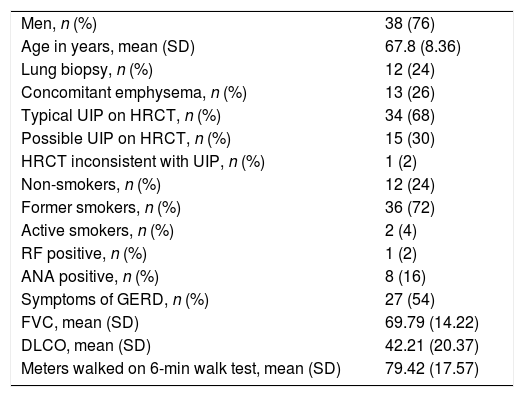
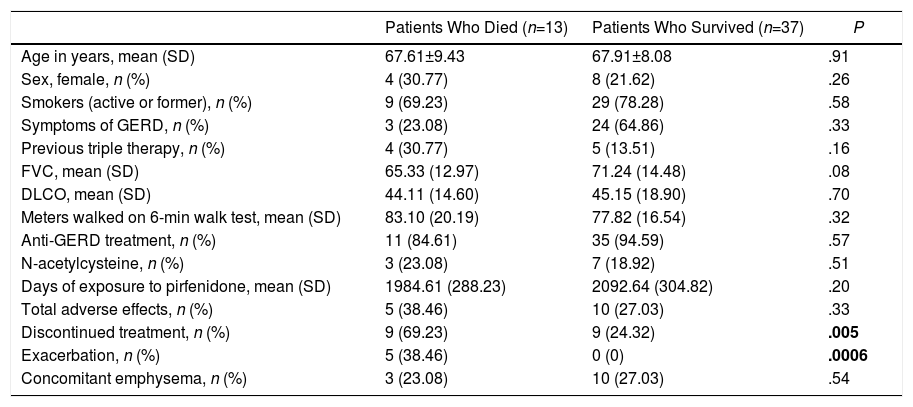
ResultsCohort DescriptionCohort characteristics are shown in Table 1. In total, 50 patients were included. The distribution of patients by hospitals was: 33 patients (66%) from the Hospital de Rehabilitación Respiratoria María Ferrer in the city of Buenos Aires, 8 patients (16%) from the Hospital Privado de Comunidad in Mar del Plata, 5 patients (10%) from the Hospital de Clínicas José de San Martin in the city of Buenos Aires, and 4 patients (8%) from the Laboratorio de Función Pulmonar de Alta Complejidad in the city of Bahía Blanca. Most patients were men (76%), with a mean (SD) age of 67.8 (8.36) years. In 12 cases (24%), surgical lung biopsy was required for diagnosis, of which 8 showed a pattern of possible UIP on CT, one had a pattern inconsistent with UIP, and 3 had a pattern of typical UIP. Thirteen patients had emphysema on HRCT, all of whom were smokers. Of the 37 remaining patients who did not show emphysema, only 12 (32.4%) had never smoked. In all cases, the diagnosis of IPF was reached after discussion among the members of the multidisciplinary committee, after carefully ruling out hidden connective tissue diseases and/or chronic hypersensitivity.14 Only 8 patients (16%) were ANA positive, and all had low titers (less than or equal to 1/160). Fourteen patients (28%) were included in the list of possible candidates for lung transplantation. Lung function parameters were recorded in 49 patients. Mean (SD) FVC at baseline was 69.7% (14.22). DLCO was measured in 37 patients, with a mean (SD) of 44.9% (77.76). PO2 was obtained in 29 patients, with a mean (SD) of 71.6mmHg (25.03). Forty-two patients performed the 6-minute walk test, with a mean (SD) of 403.69 (98.33) meters walked. Thirteen patients (26%) died during the study period, all for causes related to IPF. Five of them (38.46%) died after an exacerbation and 8 (61.53%) due to disease progression. Characteristics of patients who died were compared with those of patients who survived. Among those who died, 5 (38.46%) had an exacerbation, while none of the patients who survived presented an exacerbation. This difference was statistically significant (P=.0006). Furthermore, 9 patients (69.23%) in the group of patients who died discontinued treatment temporarily vs 9 (24.32%) in the group of patients who survived, a difference that was also statistically significant (P=.005) (Table 2). Finally, only 1 patient received single-lung transplant due to IPF progression.
Baseline Characteristics of IPF Patient Cohort Receiving Pirfenidone Treatment (n=50).
| Men, n (%) | 38 (76) |
| Age in years, mean (SD) | 67.8 (8.36) |
| Lung biopsy, n (%) | 12 (24) |
| Concomitant emphysema, n (%) | 13 (26) |
| Typical UIP on HRCT, n (%) | 34 (68) |
| Possible UIP on HRCT, n (%) | 15 (30) |
| HRCT inconsistent with UIP, n (%) | 1 (2) |
| Non-smokers, n (%) | 12 (24) |
| Former smokers, n (%) | 36 (72) |
| Active smokers, n (%) | 2 (4) |
| RF positive, n (%) | 1 (2) |
| ANA positive, n (%) | 8 (16) |
| Symptoms of GERD, n (%) | 27 (54) |
| FVC, mean (SD) | 69.79 (14.22) |
| DLCO, mean (SD) | 42.21 (20.37) |
| Meters walked on 6-min walk test, mean (SD) | 79.42 (17.57) |
ANF: anti-nuclear factor; DLCO: carbon monoxide diffusing capacity of the lung; FVC: forced vital capacity; GERD: gastroesophageal reflux disease; HRCT: high resolution computed tomography; RF: rheumatoid factor; UIP: usual interstitial pneumonia.
Comparison Between Patients Who Died (n=13) And Who Survived (n=37).
| Patients Who Died (n=13) | Patients Who Survived (n=37) | P | |
|---|---|---|---|
| Age in years, mean (SD) | 67.61±9.43 | 67.91±8.08 | .91 |
| Sex, female, n (%) | 4 (30.77) | 8 (21.62) | .26 |
| Smokers (active or former), n (%) | 9 (69.23) | 29 (78.28) | .58 |
| Symptoms of GERD, n (%) | 3 (23.08) | 24 (64.86) | .33 |
| Previous triple therapy, n (%) | 4 (30.77) | 5 (13.51) | .16 |
| FVC, mean (SD) | 65.33 (12.97) | 71.24 (14.48) | .08 |
| DLCO, mean (SD) | 44.11 (14.60) | 45.15 (18.90) | .70 |
| Meters walked on 6-min walk test, mean (SD) | 83.10 (20.19) | 77.82 (16.54) | .32 |
| Anti-GERD treatment, n (%) | 11 (84.61) | 35 (94.59) | .57 |
| N-acetylcysteine, n (%) | 3 (23.08) | 7 (18.92) | .51 |
| Days of exposure to pirfenidone, mean (SD) | 1984.61 (288.23) | 2092.64 (304.82) | .20 |
| Total adverse effects, n (%) | 5 (38.46) | 10 (27.03) | .33 |
| Discontinued treatment, n (%) | 9 (69.23) | 9 (24.32) | .005 |
| Exacerbation, n (%) | 5 (38.46) | 0 (0) | .0006 |
| Concomitant emphysema, n (%) | 3 (23.08) | 10 (27.03) | .54 |
In bold: statistically significant differences.
DLCO: carbon monoxide diffusing capacity of the lung; FVC: forced vital capacity; GERD: gastroesophageal reflux disease.
Mean (SD) number of days between onset of symptoms and IPF diagnosis was 677.7 (563.4), while the mean (SD) number of days between diagnosis and starting pirfenidone treatment was 283.40 (201.7).
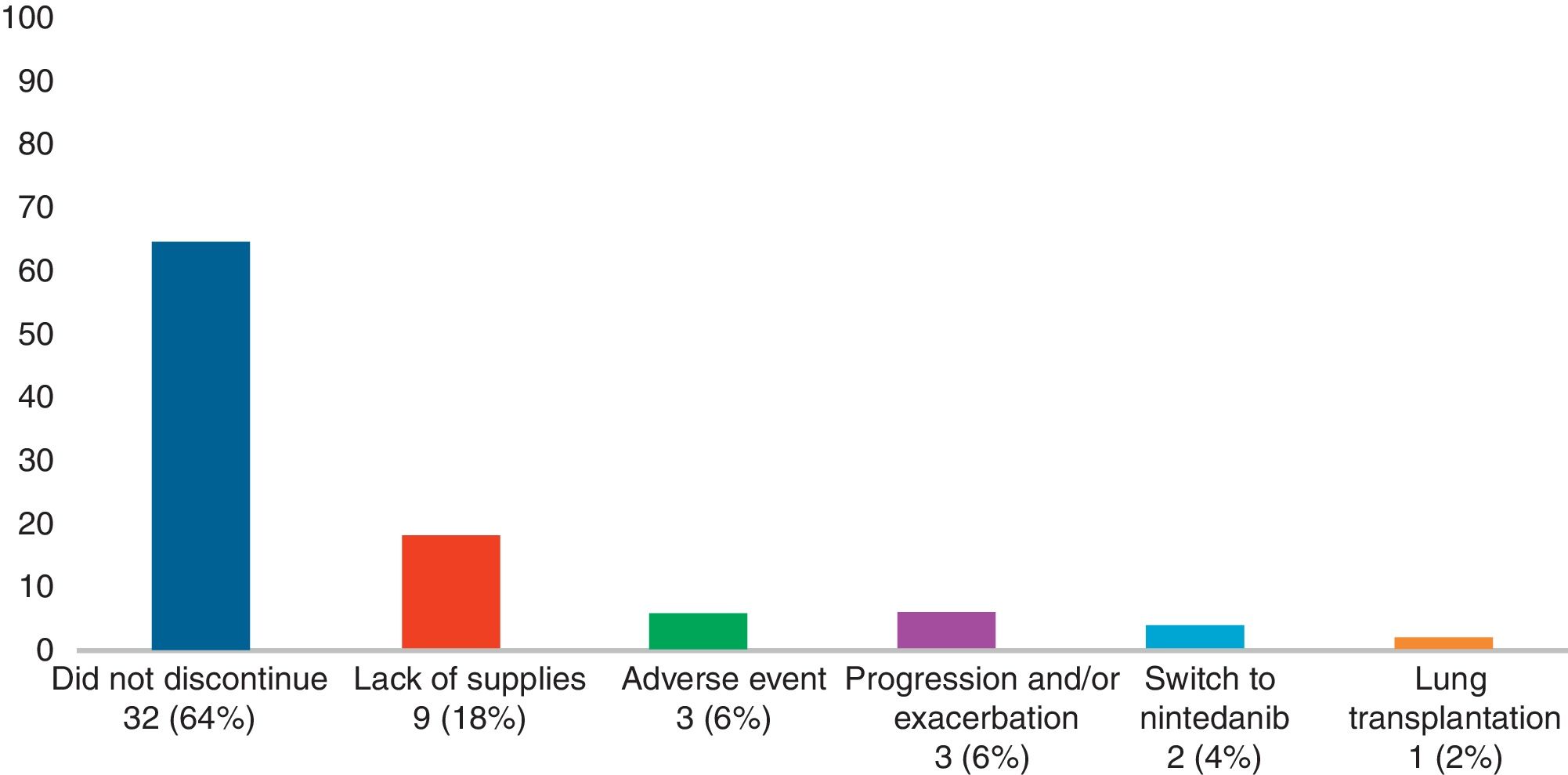
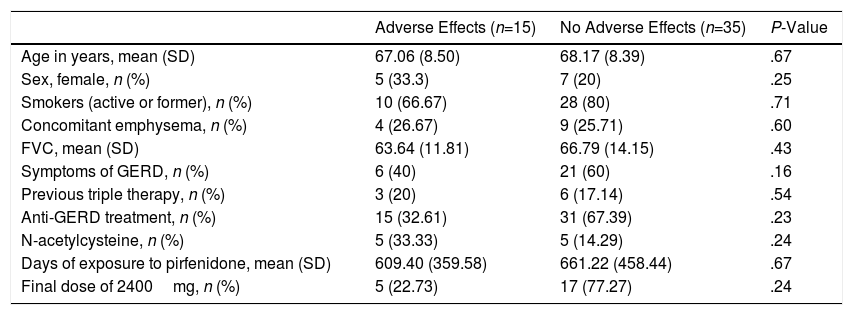
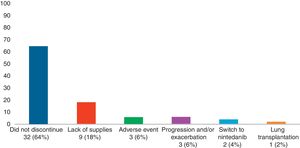
Adverse Effects AnalysisThe mean (SD) number of days of pirfenidone treatment was 645.68 (428.19), with a mean (SD) daily dose of 2064.56 (301.49)mg. Twenty-six patients (52%) received treatment with pirfenidone for longer than 1 year, and 14 (28%) for more than 2 years. Nineteen AEs were reported in a total of 15 patients (30%). Of these, 11 had only 1 adverse event and 4 patients had 2 adverse events (Table 3). Analysis of the different variables that could influence the development of AEs did not reveal statistically significant differences between both groups (Table 4). A total of 18 patients (36%) discontinued treatment: 17 discontinued temporarily for less than 1 month before resuming the full dose; and 1 discontinued definitively. Reasons for discontinuation are listed in Fig. 1. Of these 18 patients, only 3 discontinued treatment due to AEs. Two patients interrupted treatment for less than 1 month, 1 due to persistent nausea and asthenia, and the other due to severe diarrhea. The third patient discontinued definitively due to a phototoxic skin reaction involving a severe burn, requiring admission. Subsequent progress was good. This patient had a Fitzpatrick II phototype (burns easily on sun exposure and develops a light tan), predisposing him to such phototoxicity, and he failed to take the indicated protective measures. Finally, none of the patients developed hepatotoxicity.
Adverse Effects Reported in the Population of IPF Patients Receiving Treatment With Pirfenidone.
| Total adverse effects, n (%) | 15 (30) |
| Nausea, n (%) | 7 (14) |
| Phototoxic skin rash, n (%) | 4 (8) |
| Asthenia, n (%) | 5 (10) |
| Diarrhea, n (%) | 1 (2) |
| Loss of appetite, n (%) | 2 (4) |
| Significant elevation of liver enzymesa, n (%) | 0 (0) |
Incidence of Adverse Effects Among Patients Receiving Pirfenidone (n=50).
| Adverse Effects (n=15) | No Adverse Effects (n=35) | P-Value | |
|---|---|---|---|
| Age in years, mean (SD) | 67.06 (8.50) | 68.17 (8.39) | .67 |
| Sex, female, n (%) | 5 (33.3) | 7 (20) | .25 |
| Smokers (active or former), n (%) | 10 (66.67) | 28 (80) | .71 |
| Concomitant emphysema, n (%) | 4 (26.67) | 9 (25.71) | .60 |
| FVC, mean (SD) | 63.64 (11.81) | 66.79 (14.15) | .43 |
| Symptoms of GERD, n (%) | 6 (40) | 21 (60) | .16 |
| Previous triple therapy, n (%) | 3 (20) | 6 (17.14) | .54 |
| Anti-GERD treatment, n (%) | 15 (32.61) | 31 (67.39) | .23 |
| N-acetylcysteine, n (%) | 5 (33.33) | 5 (14.29) | .24 |
| Days of exposure to pirfenidone, mean (SD) | 609.40 (359.58) | 661.22 (458.44) | .67 |
| Final dose of 2400mg, n (%) | 5 (22.73) | 17 (77.27) | .24 |
DLCO: carbon monoxide diffusing capacity of the lung; FVC: forced vital capacity; GERD: gastroesophageal reflux disease.
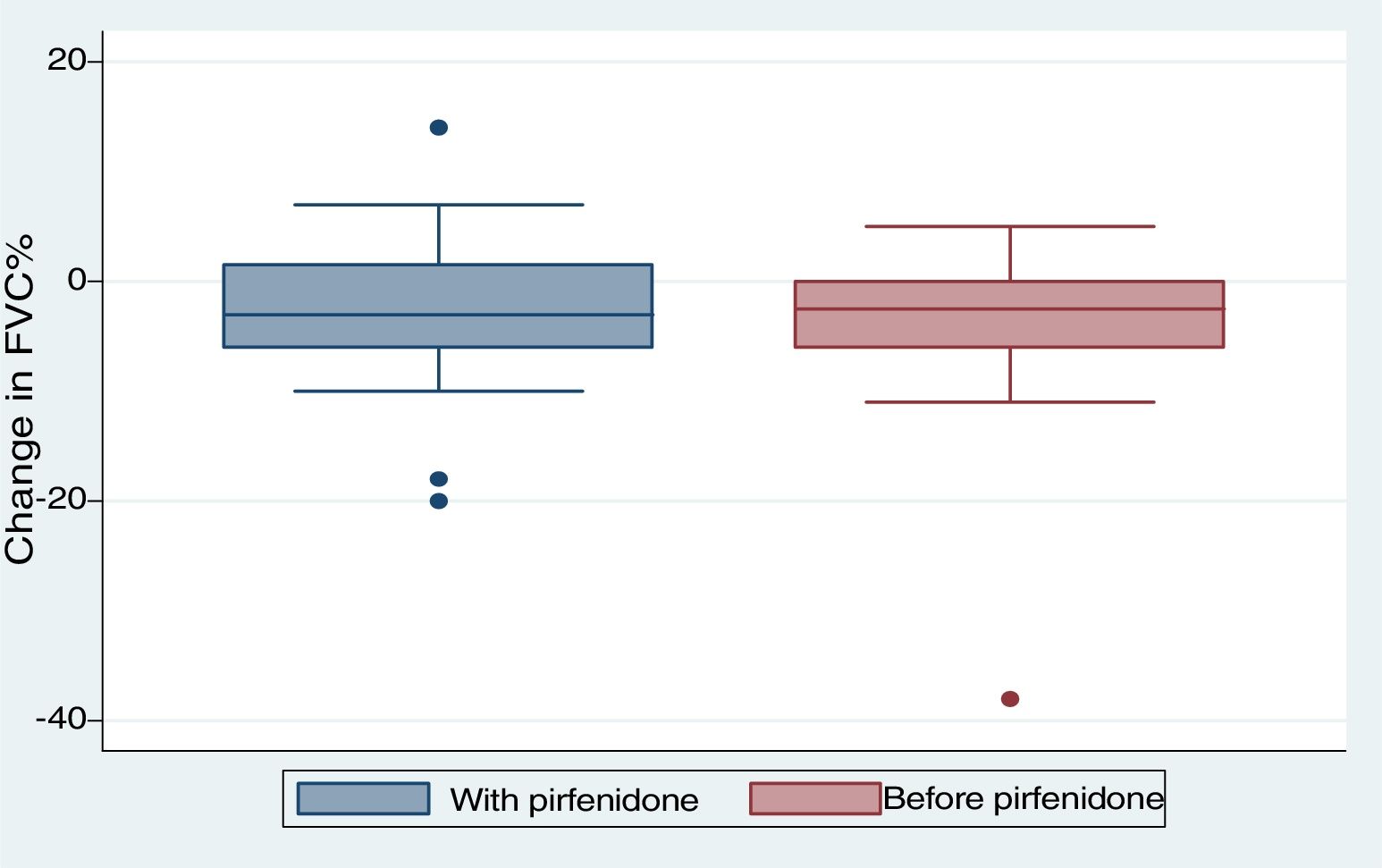
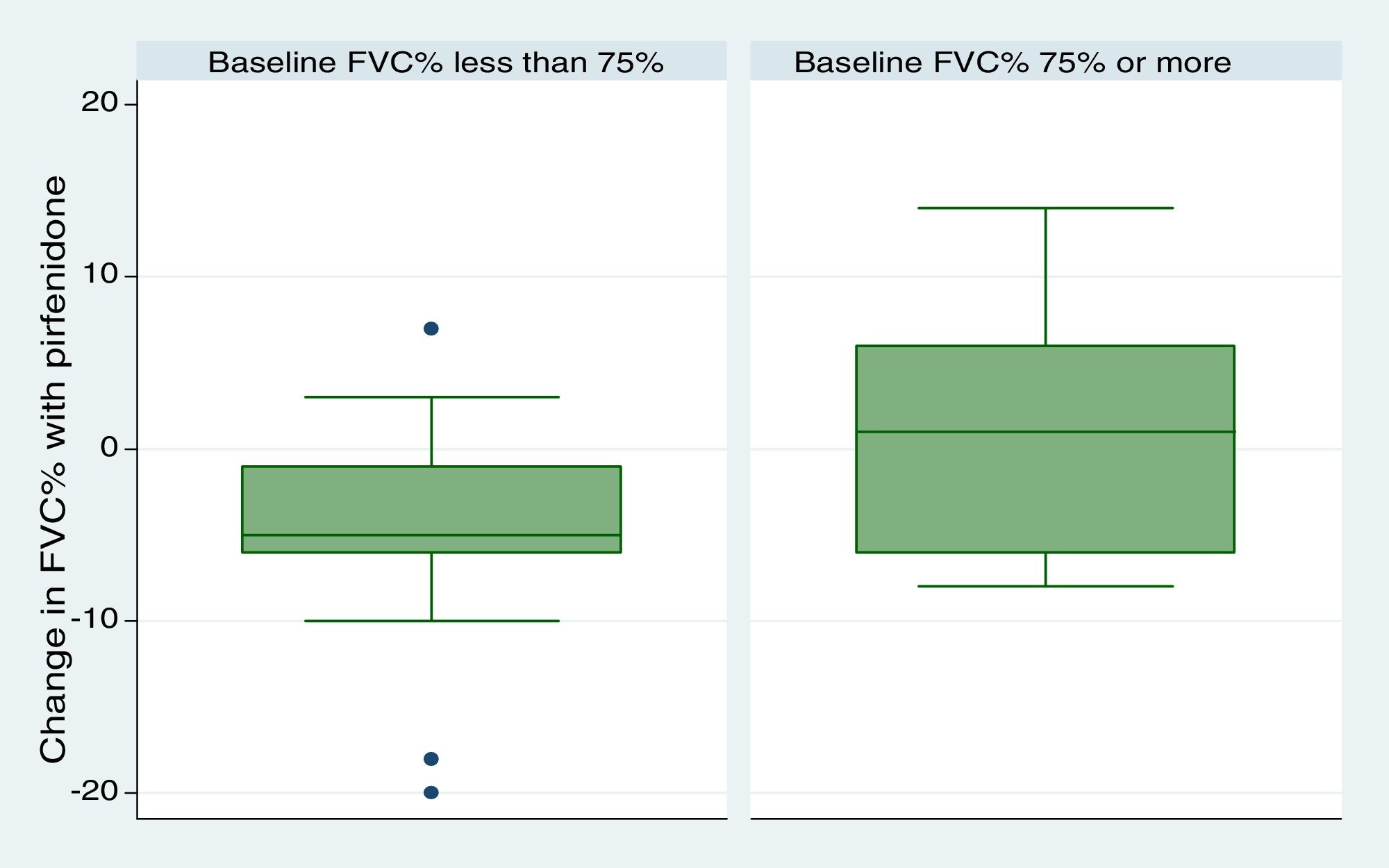
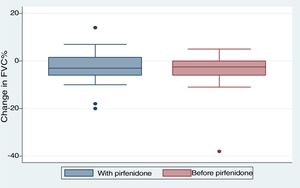
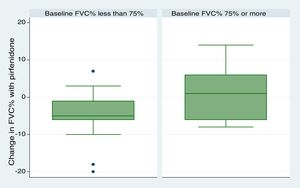
We included 28 patients in this analysis. No significant differences were found between the periods before and after starting pirfenidone. We obtained a median (IQR) functional follow-up of 173.5 days in the period before pirfenidone (127.5–265) and 172 days (144.5–270.5) in the period after starting pirfenidone (P=.247). In the comparative analysis between both periods (Fig. 2), we observed a decline in mean (SD) FVC of 4.03% (7.63) during the pre-pirfenidone period, and 2.64% (7.1) during the period after starting pirfenidone; this difference was not statistically significant (P=.534). Nor was this difference statistically significant in the subgroup of patients with concomitant emphysema. To complete this analysis, we classified the patients according to severity and compared the decline in FVC during pirfenidone treatment (Fig. 3). We found 11 patients with FVC≥75% (mild) and 17 patients with FVC<75% (moderate and severe). We found a difference in FVC behavior with a trend toward statistical significance in favor of the subgroup with FVC≥75%. This group showed a change in median (IQR) FVC of 1% (−6 to 10), compared with a median (IQR) of −5 (−6 to −1) in the <75% subgroup (P=.09). We found no differences in the proportion of patients with concomitant emphysema between both subgroups, with 3 (27.2%) in the FVC≥75% group (17.65%) and 3 in the FVC<75% group (P=.65).
In this study, we analyzed the safety and efficacy of pirfenidone in the treatment of IPF in a real-life setting. AEs occurred in 30% of the study population. As in other clinical trials, these AEs were mild with the same profile of gastrointestinal and dermatological events (14% nausea and 10% phototoxicity). Phototoxic skin rash was observed in 10% of our patients, compared with 32% and 28.1% in the ASCEND and CAPACITY studies, respectively.3,4 Fourteen percent of our patients reported experiencing nausea, while the prevalence in the CAPACITY and ASCEND studies was 36%. One reason for this could be that patients in real-life studies are less likely to report AEs compared with those participating in clinical trials, who undergo more detailed monitoring. The advanced age of our patients and their lower baseline FVC may also have affected the rate of AEs and pirfenidone tolerance. In this respect, our population was similar to those of the above-mentioned clinical trials. For example, our patients had a mean (SD) baseline FVC of 69.79% (14.22), and a mean age (SD) of 67.8 years (8.36), which was similar to those of the ASCEND study, which reported a mean (SD) FVC of 67.8% (11.2) and age of 68.4 years.6,7
With regard to discontinuation due to AEs (Fig. 1), our study figures were lower than those reported in other real-life studies: only 3 patients (6%) discontinued treatment due to AEs. In a Spanish study, 14% of participants discontinued due to AEs, while in another similar Italian study this percentage reached 16%.15,16 However, the clinical trials used to support the approval of pirfenidone in patients with IPF reported much lower drug discontinuation rates due to toxicity than real-life studies. In the CAPACITY study, only 3 patients (0.9%) had to discontinue pirfenidone due to adverse events, all cases being phototoxic burns.3,4 This demonstrates that, while the rate of reported AEs is higher in clinical trials, these events are mostly mild, and do not compromise treatment continuity. An alarming finding from our study is that the most common reason for temporary interruption of treatment was failure by providers to supply pirfenidone (generally, medical insurers). This is the first real-life study to report this problem, reflecting the difficulty experienced by both patients and physicians in maintaining pirfenidone treatment continuity in a real-life situation. We analyzed our data to determine whether any particular variable could be associated with the development of AEs (Table 3). To this end, we tested variables associated with disease severity, the use of previous or concomitant treatments, doses, and time of exposure to pirfenidone, and others. We did not find any statistically significant association between these variables and the development of AEs. Our study differs in this regard from other real-life studies, in which the administration of pirfenidone concomitantly with other drugs is associated with a greater rate of AEs than with pirfenidone alone.8 With regard to mortality, while this study was not designed to perform a time-to-event analysis, thus preventing us from analyzing survival, we did obtain interesting information on patients who died. For example, we found a greater proportion of disease exacerbations and temporary interruption of the antifibrotic therapy among non-survivors. It is important to emphasize that the most common reason for temporary interruption was the failure of providers to supply pirfenidone, thereby placing patients at risk. With regard to the effectiveness of pirfenidone, we did not observe any significant difference in FVC decline between the periods before and after starting pirfenidone (Fig. 2). It is interesting to note that the subgroup of patients with an FVC≥75% at diagnosis (mild) had a lower decline in FVC on pirfenidone, a difference that showed a trend toward statistical significance (Fig. 3). In a post hoc analysis of the CAPACITY and ASCEND clinical trials, the same efficacy was found for pirfenidone treatment in the subgroup of 146 patients with FVC≥80% as in those with lower values.3,4 Our results are also similar to those reported by the Italian group who compared the effectiveness of pirfenidone between patients with an FVC above and below 75%: a greater effect on the decline of FVC was observed in the first group.7 Taking into account that IPF is a progressive, irreversible disease, and that pirfenidone has been shown to reduce the decline in FVC over time, its greater efficacy in patients with better FVC highlights the importance of prompt diagnosis, and of starting disease-modifying therapies in early stages. Patients with emphysema on HRCT were excluded from the pivotal clinical trials. For this reason, little information is available on the behavior of pirfenidone in this subgroup. In our study, 26% of patients had emphysema on chest HRCT. These patients had the same efficacy and AE rates as patients without emphysema, although the number of patients analyzed was insufficient to draw any definitive conclusions. It is interesting that the time between the IPF diagnosis and starting pirfenidone in our study was 283.40 (201.7) days, while a real-life Italian study reported more than double this period (approximately 2 years).7 One reason may be that all hospitals that participated in our study had specialist IPF teams, a factor that might facilitate access to medication. In contrast, the time between onset of symptoms and diagnosis of the disease was 677.7 (563.4) days. This reflects the delay in referral to a specialized center, and is important, since it has been demonstrated that such a delay is directly related to increased mortality.17 Our study has some limitations. It is a retrospective observational study, so a selection bias cannot be ruled out. The possibility of this bias was minimized by the consecutive inclusion of all patients seen in the participating hospitals. As this was a retrospective real-life study, each of the participating centers used a different form or clinical history for data collection. This could lead to information bias, or more specifically, reporting bias. With regard to functional follow-up, we could only compare progress between the periods before and after the start of pirfenidone treatment in 28 of the 50 patients. This is because the follow-up times of each period were very different in the remaining patients. Although this fact prevented us from studying the efficacy of the drug, this was not the primary objective of the study. Despite these limitations, we assume that our results are valid due to the fact that this is a non-sponsored multicenter study. The differences between our study and other real-life studies underlines the importance of analyses of this type in different populations. Our results demonstrate good tolerance of pirfenidone in IPF patients, supporting the results seen in clinical trials and other real-life observational studies. With regard to the effectiveness of pirfenidone, we did not observe any significant difference in FVC decline between the periods before and after starting pirfenidone.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Caro FM, Alberti ML, Campins F, Enghelmayer JI, Fernández ME, Lancellotti D, et al. Experiencia de la vida real con pirfenidona en la fibrosis pulmonar idiopática en Argentina. Estudio retrospectivo multicéntrico. Arch Bronconeumol. 2019;55:75–80.