Bronchiectasis is a very heterogeneous disease but some homogeneous groups with similar clinical characteristics and prognosis have been identified. Exacerbations have been shown to have a negative impact on the natural history of bronchiectasis. The objective of this study was to identify the definition and characteristics of the “frequent exacerbator patient” with the best prognostic value and its relationship with the severity of bronchiectasis.
MethodsA historical cohort of 651 patients diagnosed with bronchiectasis was included. They had all received 5 years of follow-up since their radiological diagnosis. Exacerbation was defined as a worsening of the symptoms derived from bronchiectasis that required antibiotic treatment. The main outcome was all-cause mortality at the end of follow-up.
ResultsThe mean age was 48.2 (16) years (32.9% males). 39.8% had chronic infection by Pseudomonas aeruginosa. Mean BSI, FACED, and E-FACED were 7 (4.12), 2.36 (1.68), and 2.89 (2.03), respectively. There were 95 deaths during follow-up. The definition of the “frequent exacerbator patient” that presented the greatest predictive power for mortality was based on at least two exacerbations/year or one hospitalization/year (23.3% of patients; AUC-ROC: 0.75 [95% CI: 0.69–0.81]). Its predictive power was independent of the patient's initial severity. The clinical characteristics of the frequent exacerbator patient according to this definition varied according to the initial severity of bronchiectasis, presence of systemic inflammation, and treatment.
ConclusionsThe combination of two exacerbations or one hospitalization per year is the definition of frequent exacerbator patient that has the best predictive value of mortality independent of the initial severity of bronchiectasis.
Las bronquiectasias son una enfermedad muy heterogénea en la que se han identificado algunos grupos homogéneos con características clínicas y pronóstico similares. El objetivo de este estudio fue establecer la definición y características del “paciente exacerbador frecuente” que presenta mejor valor pronóstico y su relación con la gravedad de las bronquiectasias.
MétodosSe analizó una cohorte histórica de 651 pacientes diagnosticados de bronquiectasias. Se siguió a todos ellos durante cinco años desde su diagnóstico radiológico. La exacerbación se definió como un empeoramiento de los síntomas de las bronquiectasias para el que se requiera tratamiento antibiótico. El principal resultado analizado fue la mortalidad por todas las causas al final del seguimiento.
ResultadosLa edad media fue 48,2 (16) años (39,2% de hombres). El 38,9% tuvo infección por Pseudomonas aeruginosa. Los valores medios de BSI, FACED y E-FACED fueron 7 (4,12), 2,36 (1,68) y 2,89 (2,03), respectivamente. Hubo 96 muertes durante el seguimiento. La definición de “paciente exacerbador frecuente” que presentó el mayor valor predictivo para la mortalidad incluía la aparición de al menos dos exacerbaciones/año o un ingreso hospitalario/año (23.3% de los pacientes; AUC-ROC:0.75 [IC 95%: 0.69–0.81]). Su valor predictivo fue independiente de la gravedad inicial del paciente. Las características clínicas del “paciente exacerbador frecuente”, de acuerdo con esta definición, variaron según la gravedad inicial de la bronquiectasia, la presencia de inflamación sistémica y el tratamiento.
ConclusionesLa combinación de dos exacerbaciones o un ingreso hospitalario al año constituye la mejor definición de “paciente exacerbador frecuente” con mayor valor predictivo para la mortalidad, independientemente de la gravedad inicial de las bronquiectasias.
Bronchiectasis is a very heterogeneous disease as regards both its clinical presentation and its prognosis and treatment. This has led some authors to suggest the existence of various phenotypes based on patients’ clinical characteristics.1–4
Exacerbations have been shown to have a negative impact on the natural history of bronchiectasis patients.5–10 Thus, both the number and, more particularly, the severity of exacerbations have been associated with more serious forms of the disease, 11–15 lower quality of life, 16,17 chronic bronchial infection by potentially pathogenic microorganisms,18 rapid decline in pulmonary function,19 greater systemic inflammation,20 increased costs, 21–23 and premature death.11–14,24,25 This situation has prompted many of the published and validated multidimensional scores that quantify the severity of bronchiectasis to include exacerbations among their variables and even assign them a high predictive power.11,15 However, these scores are mostly comprised of variables recorded close to the date of the diagnosis of bronchiectasis and so, despite their high predictive power, they do not cover circumstances that arise over the course of the patient's follow-up.
Those patients with bronchiectasis and multiple exacerbations could constitute a special group of patients with their own clinical, prognostic, and therapeutic characteristics known as “frequent exacerbator patients” or “exacerbator clinical phenotype”,25 and it has already been observed and recognized in other airway diseases such as COPD.26 Various definitions of the “frequent exacerbator patient” in bronchiectasis can be found in the literature, based on the combination of the annual rate of exacerbations and hospitalizations. However, there is very little literature on this group of bronchiectasis patients with multiple exacerbations, since only one very recent study has adequately assessed its characteristics and prognostic value.25
The objective of this study was to analyze the relationship between the presence of multiple exacerbations/hospitalizations and mortality and to identify the definition of the “frequent exacerbator patient” in bronchiectasis that offers the best prognostic value for mortality, as well as its relationship with the initial severity of the bronchiectasis (as measured by the various published multidimensional scores).
Material and MethodsDesignObservational, multi-center study of a large historical cohort involving six teaching centers in Latin America, all with multidisciplinary, protocolized specialist bronchiectasis outpatient clinics.
PatientsThis study drew on the Latin American bronchiectasis database, which covers six series from six Latin American hospitals (four in Brazil, one in Argentina and one in Chile), with a total of 651 bronchiectasis patients who were the subjects for the external validation of the FACED14 and E-FACED15 scores. All the patients were diagnosed as having clinically active non-cystic fibrosis bronchiectasis at a primary diagnosis using high-resolution computed tomography (HRCT),27 and they presented a wide range of severity, etiologies, clinical pictures and functional impairments. Patients aged under 18 years were excluded, along with those whose vital state was unknown at the end of the follow-up. This study was approved by the Ethics and Research Committee of each participating center.
MethodsApart from the patients’ baseline characteristics at the time of their diagnosis, this cohort offers longitudinal data collected during the subsequent 5 years of follow-up, related to the number of annual exacerbations and hospitalizations, the administration of chronic treatments, and microbiological findings (isolation of potentially pathogenic microorganisms). The exacerbations were recorded in the hospital databases. Exacerbation was defined as a worsening of the respiratory symptoms, concomitant with an increase in the volume or purulence of sputum that requires antibiotic treatment.
Description of the Multidimensional Grading SystemsThe FACED score consists of five dichotomic variables: FEV1, age, presence of chronic infection by Pseudomonas aeruginosa, dyspnea scale (mMRC), and number of pulmonary lobes affected on computed tomography.28 E-FACED adds the number of hospitalizations due to an exacerbation in the previous year (E).15 The possible range of points is 0–7 (FACED) and 0–9 (E-FACED). The division of the FACED/E-FACED scores into three groups makes it possible to define bronchiectasis as mild (0–2 points [FACED]/0–3 points [E-FACED]), moderate (3–4 points [FACED]/4–6 points [E-FACED]), and severe (5–7 points [FACED]/7–9 points [E-FACED]).
Bronchiectasis severity index (BSI)11 consists in nine variables: those included in E-FACED score plus body mass index, chronic infection by other pathogenic microorganisms different from P. aeruginosa, and number of exacerbations with a range of 0–26 points. An overall score is derived as a sum of the scores for each variable and it may range from 0 to 26 points. According to the overall score value, the patients with bronchiectasis are classified into three BSI classes: patients with low BSI score (overall score 0–4 points), patients with intermediate BSI score (overall score 5–8 points), and patients with high BSI score (overall score 9 or more points).
Follow-upThe patients were followed up for 5 years after the radiological diagnosis of bronchiectasis. After these 5 years, their vital state was determined. The number of deaths, and their causes, was established via the hospitals’ computerized records or the corresponding death certificates.
Main OutcomeThe main outcome was the prognostic capacity for all-cause mortality of the best definition of frequent exacerbator patient, based on the mean annual frequency and severity of exacerbations.
Statistical AnalysisThe data were tabulated as mean/median or standard deviation/interquartile range in the case of quantitative variables. Qualitative variables were tabulated as absolute value and percentage of the total. Although a risk period to assess the risk factor was not established prior to measuring the outcome, a sample size calculation was performed to demonstrate that the inclusion in the study of 651 patients with a mean annual frequency of exacerbations (including hospitalizations due to bronchiectasis exacerbations) of 1.25 per year and 5 years follow-up were appropriate to perform all the analyses of this study with an adequate statistical power (an α error of 5%, and a statistical power of 80%).
AUC-ROC curves (95% CI) were used to identify the prognostic value of the presence of different numbers of exacerbations (≥1, ≥2, ≥3 per year) and/or hospitalizations ((≥1, ≥2, ≥3 per year), as well as the prognostic value of the best combination of the number of exacerbations and/or hospitalizations. Those definitions with an AUC-ROC>0.70 would then be selected for further analysis. Bivariate analyses were undertaken using the Student's t-test, Mann–Whitney U-test, or Chi-square test, depending on the distribution of the variables. The Kaplan–Meier curve was used to compare the two groups (frequent exacerbator versus non-frequent exacerbator patients).
In order to determine the relationship between the presence of a frequent exacerbator phenotype (definitions with an AUC-ROC>0.70) and the initial severity of bronchiectasis, and mortality from any cause at 5 years, both variables (severity scores and presence of frequent exacerbator definition as a dichotomic variable) were introduced into different Cox multivariate survival models with mortality from any cause at 5 years of follow-up as the dependent cause. The strength of the association was measured via the HR (95% CI). Bonferroni test was used to correct the p-values for multiple comparisons.
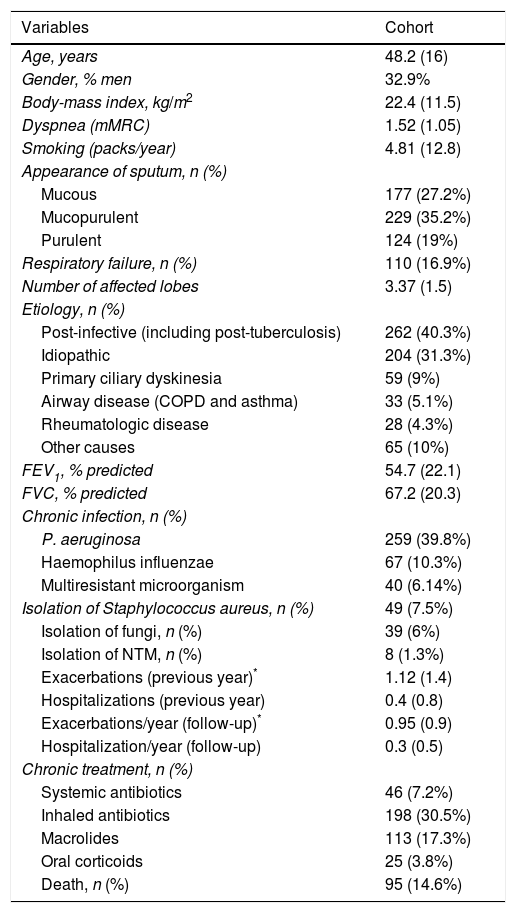
ResultsSix hundred and fifty-one patients were included, taken from six Latin American hospitals. Data were not obtained from 21 patients (3.1%), mainly because of lack of information on their vital status at the end of the study. The mean age was 48.2 (16) years, 32.9% were men, and 39.8% presented chronic colonization by P. aeruginosa. The mean BSI, FACED, E-FACED scores were 7 (4.12), 2.36 (1.68), and 2.89 (2.03) respectively. The mean FEV1 was 54.7% (22.1). There were 95 (14.6%) deaths during the 5 years of the follow-up. The main cause of death was related to respiratory complications (66%), cardiovascular disease (18%), and cancer (10%). The mean number of exacerbations per year was 0.95 (0.9) (not including hospitalizations), while the number of hospitalizations per year was 0.4 (0.5) (Table 1).
Baseline Characteristics of the Included Patients (n=651).
| Variables | Cohort |
|---|---|
| Age, years | 48.2 (16) |
| Gender, % men | 32.9% |
| Body-mass index, kg/m2 | 22.4 (11.5) |
| Dyspnea (mMRC) | 1.52 (1.05) |
| Smoking (packs/year) | 4.81 (12.8) |
| Appearance of sputum, n (%) | |
| Mucous | 177 (27.2%) |
| Mucopurulent | 229 (35.2%) |
| Purulent | 124 (19%) |
| Respiratory failure, n (%) | 110 (16.9%) |
| Number of affected lobes | 3.37 (1.5) |
| Etiology, n (%) | |
| Post-infective (including post-tuberculosis) | 262 (40.3%) |
| Idiopathic | 204 (31.3%) |
| Primary ciliary dyskinesia | 59 (9%) |
| Airway disease (COPD and asthma) | 33 (5.1%) |
| Rheumatologic disease | 28 (4.3%) |
| Other causes | 65 (10%) |
| FEV1, % predicted | 54.7 (22.1) |
| FVC, % predicted | 67.2 (20.3) |
| Chronic infection, n (%) | |
| P. aeruginosa | 259 (39.8%) |
| Haemophilus influenzae | 67 (10.3%) |
| Multiresistant microorganism | 40 (6.14%) |
| Isolation of Staphylococcus aureus, n (%) | 49 (7.5%) |
| Isolation of fungi, n (%) | 39 (6%) |
| Isolation of NTM, n (%) | 8 (1.3%) |
| Exacerbations (previous year)* | 1.12 (1.4) |
| Hospitalizations (previous year) | 0.4 (0.8) |
| Exacerbations/year (follow-up)* | 0.95 (0.9) |
| Hospitalization/year (follow-up) | 0.3 (0.5) |
| Chronic treatment, n (%) | |
| Systemic antibiotics | 46 (7.2%) |
| Inhaled antibiotics | 198 (30.5%) |
| Macrolides | 113 (17.3%) |
| Oral corticoids | 25 (3.8%) |
| Death, n (%) | 95 (14.6%) |
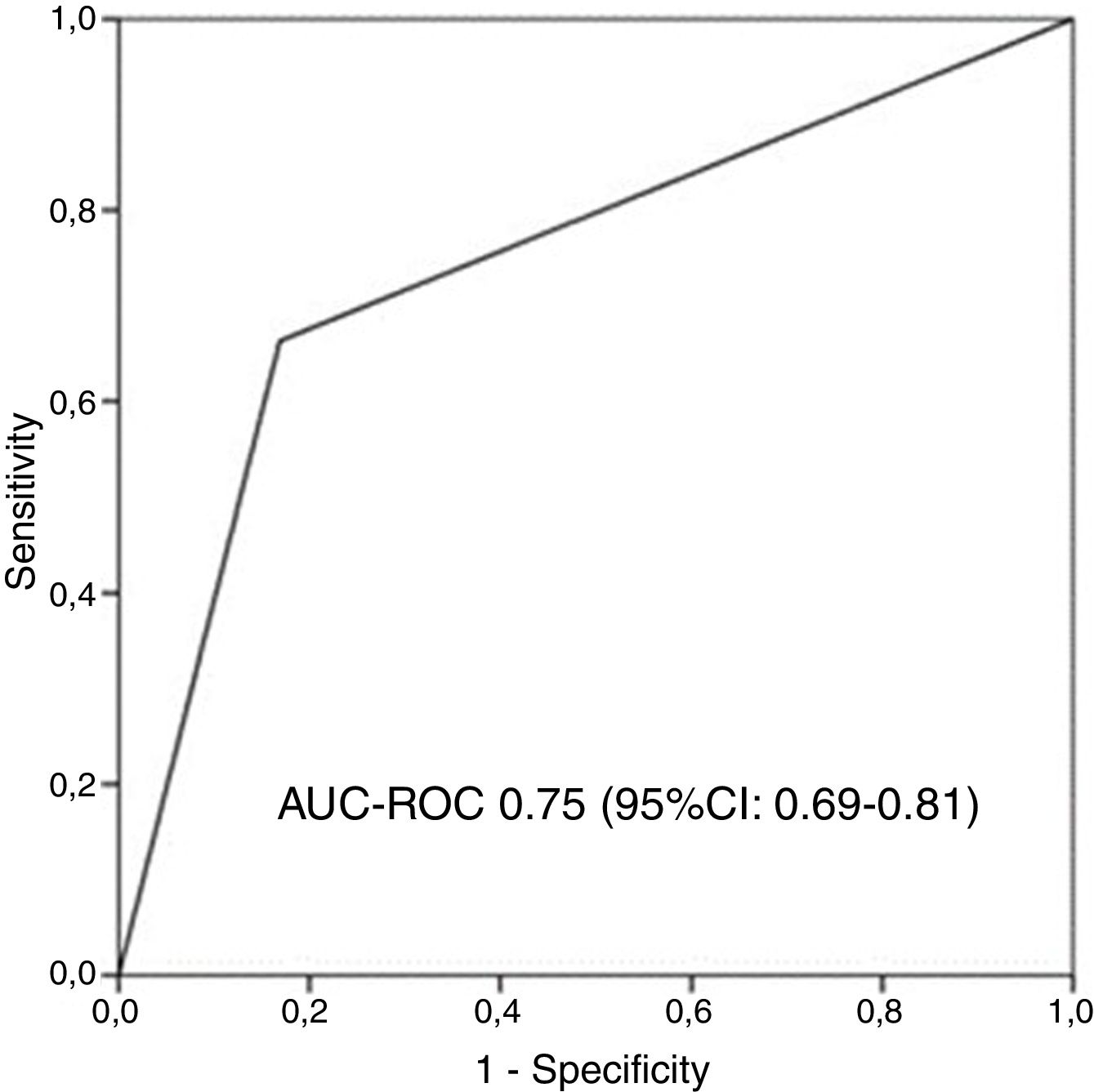
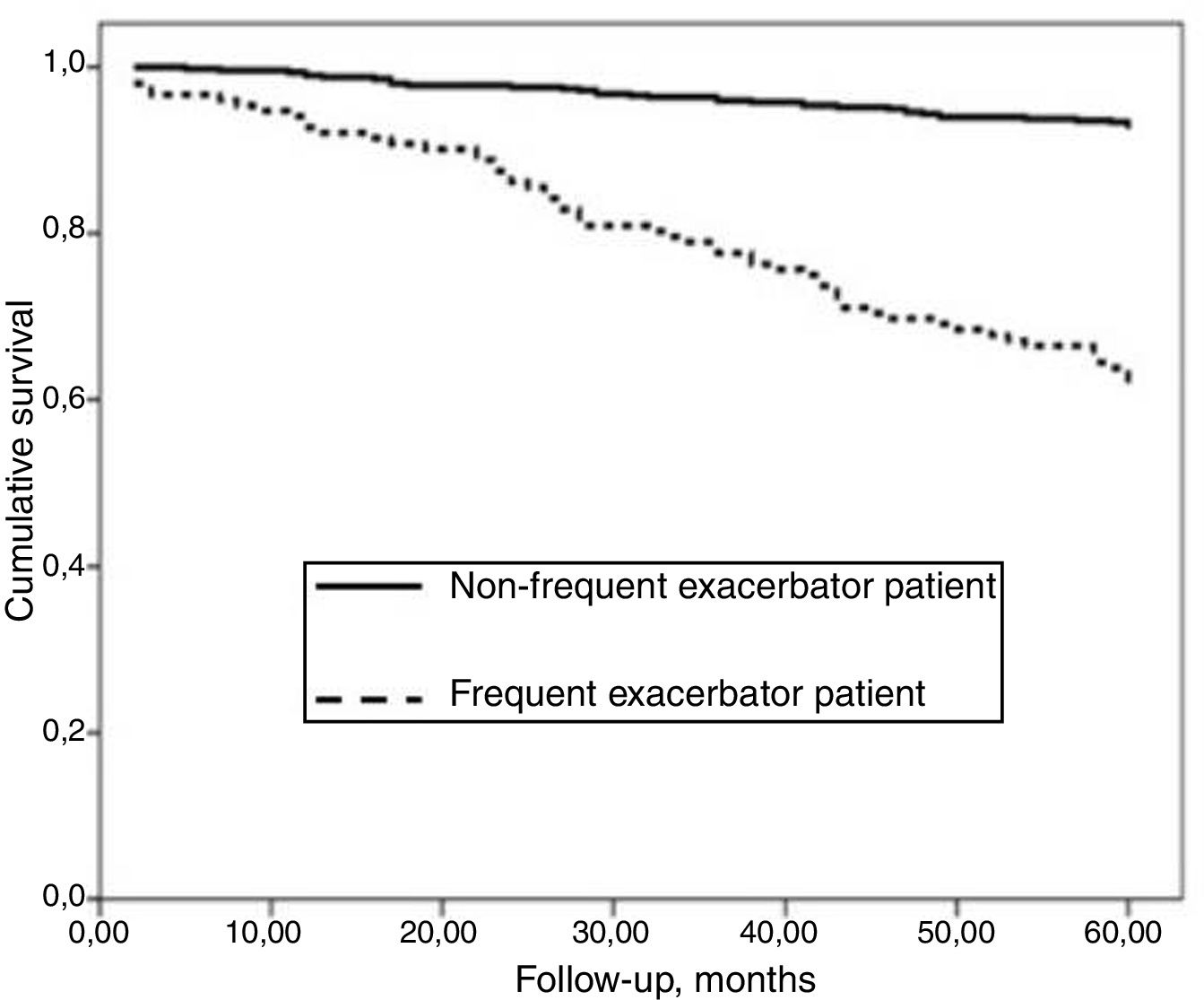
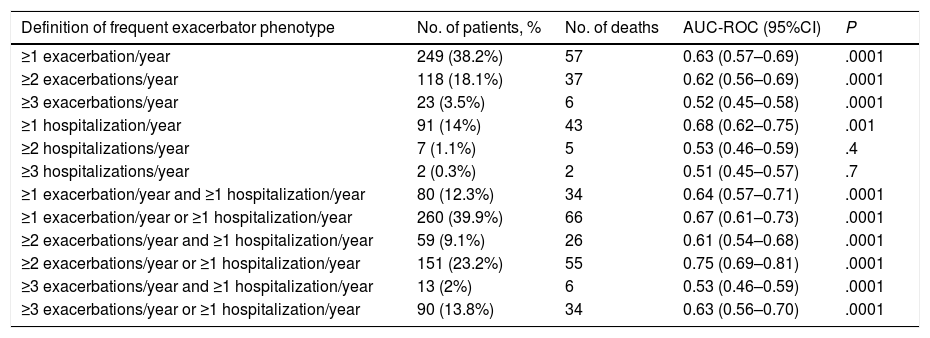
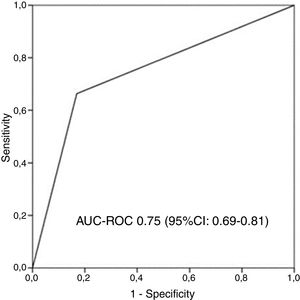
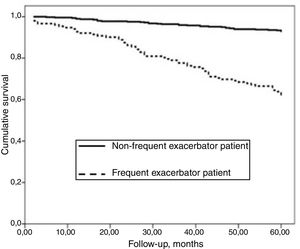
AUC-ROC (95% CI) of 12 different potential definitions of frequent exacerbator patient, based on the combination of different numbers of exacerbations and hospitalizations are showed in Table 2. The definition with the best prognostic value of 5-year all-cause mortality was “at least two exacerbations or one hospitalization/year” (23.2% of patients), which was the only one that exceeded an AUC>0.70 (AUC-ROC: 0.75 [0.69–0.81], Fig. 1). 27% of patients fulfilled this criterion taking into account only the number/severity of exacerbations in the year prior to the inclusion in the study. Fig, 2 shows the Kaplan–Meier curve using this selected definition of the frequent exacerbator patient.
Prognostic Value of 5-year All-cause Mortality (AUC-ROC) of 12 Different Potential Definitions of “Frequent Exacerbator Patient” in Bronchiectasis.
| Definition of frequent exacerbator phenotype | No. of patients, % | No. of deaths | AUC-ROC (95%CI) | P |
|---|---|---|---|---|
| ≥1 exacerbation/year | 249 (38.2%) | 57 | 0.63 (0.57–0.69) | .0001 |
| ≥2 exacerbations/year | 118 (18.1%) | 37 | 0.62 (0.56–0.69) | .0001 |
| ≥3 exacerbations/year | 23 (3.5%) | 6 | 0.52 (0.45–0.58) | .0001 |
| ≥1 hospitalization/year | 91 (14%) | 43 | 0.68 (0.62–0.75) | .001 |
| ≥2 hospitalizations/year | 7 (1.1%) | 5 | 0.53 (0.46–0.59) | .4 |
| ≥3 hospitalizations/year | 2 (0.3%) | 2 | 0.51 (0.45–0.57) | .7 |
| ≥1 exacerbation/year and ≥1 hospitalization/year | 80 (12.3%) | 34 | 0.64 (0.57–0.71) | .0001 |
| ≥1 exacerbation/year or ≥1 hospitalization/year | 260 (39.9%) | 66 | 0.67 (0.61–0.73) | .0001 |
| ≥2 exacerbations/year and ≥1 hospitalization/year | 59 (9.1%) | 26 | 0.61 (0.54–0.68) | .0001 |
| ≥2 exacerbations/year or ≥1 hospitalization/year | 151 (23.2%) | 55 | 0.75 (0.69–0.81) | .0001 |
| ≥3 exacerbations/year and ≥1 hospitalization/year | 13 (2%) | 6 | 0.53 (0.46–0.59) | .0001 |
| ≥3 exacerbations/year or ≥1 hospitalization/year | 90 (13.8%) | 34 | 0.63 (0.56–0.70) | .0001 |
Receiver operating characteristic curve and area under the curve (AUC) to determine the overall predictive value of all-cause mortality after 5 years of follow-up of the chosen definition of “frequent exacerbator patient” (at least two exacerbations per year or at least one hospitalization per year). AUC-ROC: area under curve-ROC.
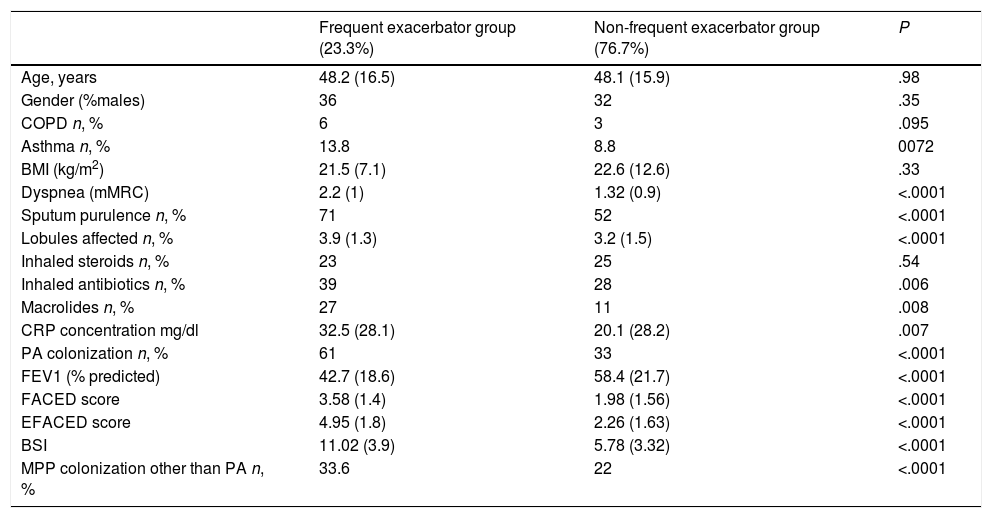
Those patients who presented annual multiple exacerbations in keeping with the agreed definition presented a greater initial severity, whichever multidimensional score was used, as well as more systemic inflammation and a greater use of antibiotic and anti-inflammatory therapies. No differences were observed, however, in age, gender, or respiratory comorbidities, although there was a statistically non-significant tendency for patients to present COPD or asthma as a secondary diagnosis (Table 3).
Comparison of the Characteristics of Patients Who Satisfied the Definition of the Chosen Frequent Exacerbator Patient and Those of the Other Patients With Bronchiectasis.
| Frequent exacerbator group (23.3%) | Non-frequent exacerbator group (76.7%) | P | |
|---|---|---|---|
| Age, years | 48.2 (16.5) | 48.1 (15.9) | .98 |
| Gender (%males) | 36 | 32 | .35 |
| COPD n, % | 6 | 3 | .095 |
| Asthma n, % | 13.8 | 8.8 | 0072 |
| BMI (kg/m2) | 21.5 (7.1) | 22.6 (12.6) | .33 |
| Dyspnea (mMRC) | 2.2 (1) | 1.32 (0.9) | <.0001 |
| Sputum purulence n, % | 71 | 52 | <.0001 |
| Lobules affected n, % | 3.9 (1.3) | 3.2 (1.5) | <.0001 |
| Inhaled steroids n, % | 23 | 25 | .54 |
| Inhaled antibiotics n, % | 39 | 28 | .006 |
| Macrolides n, % | 27 | 11 | .008 |
| CRP concentration mg/dl | 32.5 (28.1) | 20.1 (28.2) | .007 |
| PA colonization n, % | 61 | 33 | <.0001 |
| FEV1 (% predicted) | 42.7 (18.6) | 58.4 (21.7) | <.0001 |
| FACED score | 3.58 (1.4) | 1.98 (1.56) | <.0001 |
| EFACED score | 4.95 (1.8) | 2.26 (1.63) | <.0001 |
| BSI | 11.02 (3.9) | 5.78 (3.32) | <.0001 |
| MPP colonization other than PA n, % | 33.6 | 22 | <.0001 |
COPD, chronic obstructive pulmonary disease; BMI, body mass index; MRC, Medical Research Council; CRP, C-reactive protein; PA, P. aeruginosa; FEV1, forced expiratory volume in the first second; BSI, bronchiectasis severity index; PPM, potentially pathogenic microorganisms.
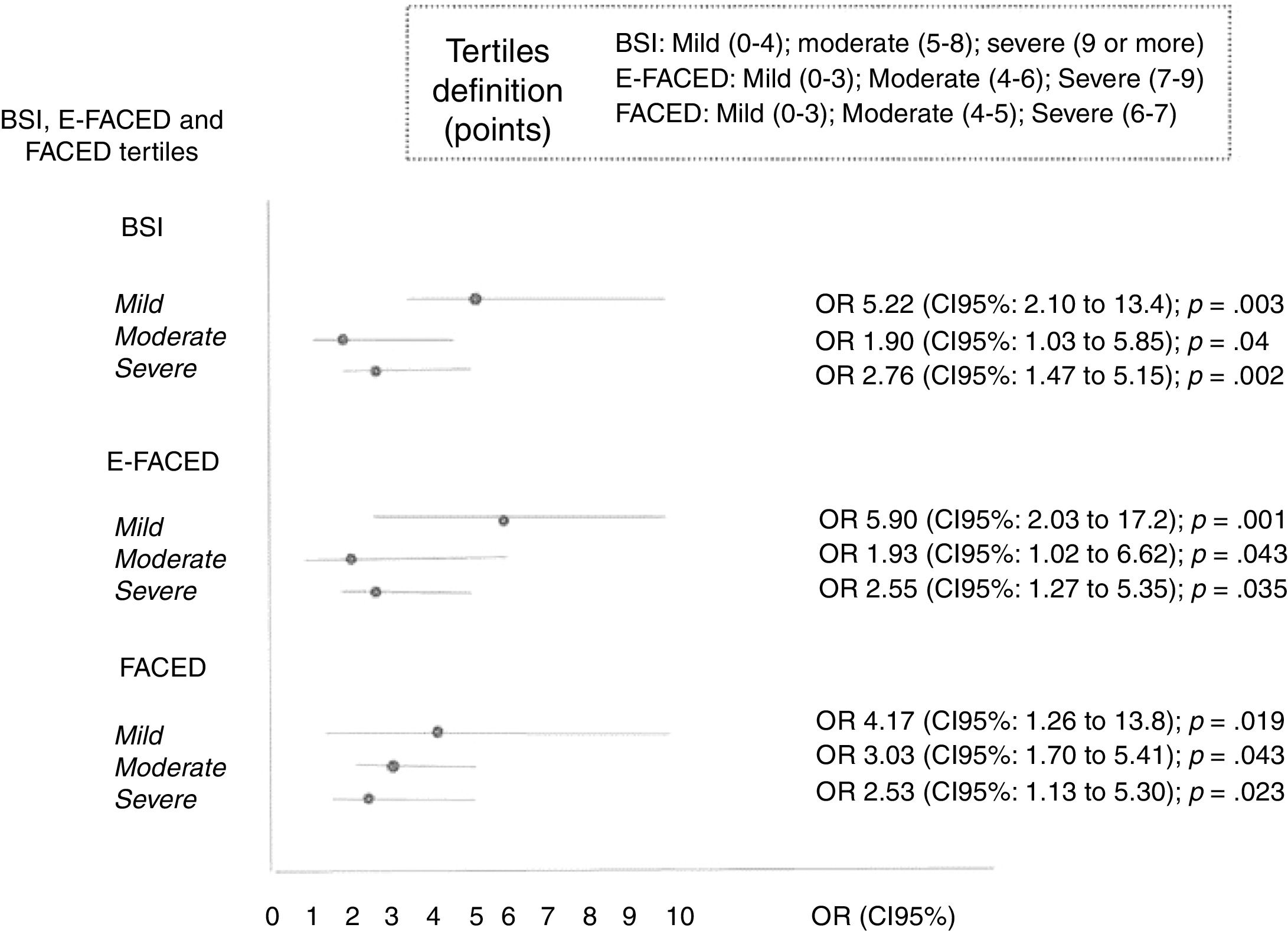
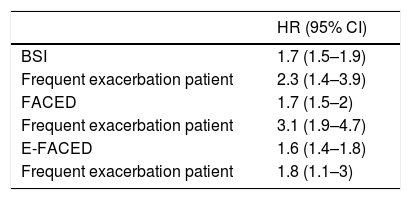
Selected definitions of frequent exacerbator patients were introduced as an independent variables in three multivariate Cox analyses, where combined with each of the three published multidimensional scores with external validation (BSI, FACED, and E-FACED) (Table 4). It can be seen that regardless of the score used to evaluate the initial severity of bronchiectasis, the presence of at least two exacerbations per year or one hospitalization per year as a consequence of bronchiectasis is independently related to a higher all-cause mortality.
Cox Regressions Using Different Combinations of Multidimensional Severity Scores as Independent Variables to Evaluate the Initial Severity of Bronchiectasis, and the Selected Definition of Frequent Exacerbator Patient.
| HR (95% CI) | |
|---|---|
| BSI | 1.7 (1.5–1.9) |
| Frequent exacerbation patient | 2.3 (1.4–3.9) |
| FACED | 1.7 (1.5–2) |
| Frequent exacerbation patient | 3.1 (1.9–4.7) |
| E-FACED | 1.6 (1.4–1.8) |
| Frequent exacerbation patient | 1.8 (1.1–3) |
At least two exacerbations per year of follow-up, or at least one hospitalization per year of follow-up (23.3%). The exacerbation variables included in the BSI and E-FACED refer only to those that occurred in the year previous to the radiological diagnosis of bronchiectasis, so these exacerbations are not included in the definition of “exacerbator patient”. The FACED score does not include any variable referring to exacerbations.
There was a significant correlation between the number of exacerbations during the year prior to inclusion in the study (as a part of the E-FACED and BSI scores), and the number of exacerbations per year during the follow-up (r=0.3; p=0.001 for exacerbations, and r=0.35, p=0.001 for hospitalizations). Although this correlation was only weak and should be not considered a clear collinearity, we repeated all the analyses after removing the number of exacerbations in the previous year from the BSI and E-FACED scores and the results did not change.
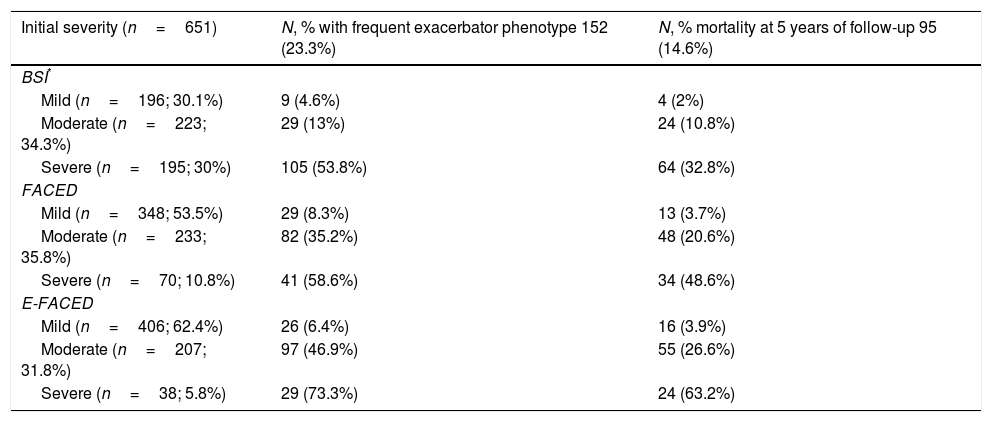
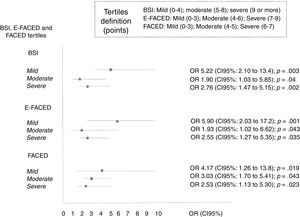
The percentage of patients with the frequent exacerbator phenotype and associated mortality according to the severity of the bronchiectasis (mild, moderate, or severe), as evaluated by the different multidimensional scores is showed in Table 5. The presence of frequent exacerbations, in the terms of the proposed definition, presents a prognostic capacity for mortality, regardless of the patient's initial severity in each of the subgroups (mild, moderate, or severe) of the BSI, FACED, and E-FACED, with similar behavior in these three multidimensional scores (Fig. 3).
Distribution of Patients With Frequent Exacerbation, Using the Selected Definition, and Associated Mortality According to the Severity (Mild, Moderate or Severe) Evaluated by the BSI, FACED, and E-FACED Scores.
| Initial severity (n=651) | N, % with frequent exacerbator phenotype 152 (23.3%) | N, % mortality at 5 years of follow-up 95 (14.6%) |
|---|---|---|
| BSI* | ||
| Mild (n=196; 30.1%) | 9 (4.6%) | 4 (2%) |
| Moderate (n=223; 34.3%) | 29 (13%) | 24 (10.8%) |
| Severe (n=195; 30%) | 105 (53.8%) | 64 (32.8%) |
| FACED | ||
| Mild (n=348; 53.5%) | 29 (8.3%) | 13 (3.7%) |
| Moderate (n=233; 35.8%) | 82 (35.2%) | 48 (20.6%) |
| Severe (n=70; 10.8%) | 41 (58.6%) | 34 (48.6%) |
| E-FACED | ||
| Mild (n=406; 62.4%) | 26 (6.4%) | 16 (3.9%) |
| Moderate (n=207; 31.8%) | 97 (46.9%) | 55 (26.6%) |
| Severe (n=38; 5.8%) | 29 (73.3%) | 24 (63.2%) |
According to our results, the presence of frequent exacerbations has a prognostic value for overall mortality after 5 years of follow-up that is independent of the initial severity of the bronchiectasis. This prognostic capacity is similarly independent of the multidimensional score system used to evaluate the initial disease severity.
One of the most important, but possibly least investigated, aspects of bronchiectasis are its exacerbations, as these have been shown to influence patients’ prognosis, especially in the more severe forms of the disease, independent of any other variables.11–20,25 Accordingly, several published and validated scores of severity have chosen to incorporate exacerbations as a variable. Chalmers et al. observed that both the number and severity of the exacerbations experienced in the year prior to the diagnosis were an independent risk factor for various major parameters, such as quality of life, mortality, and some biomarkers.11 Furthermore, some of the scores of severity have demonstrated a predictive capacity for those patients with a greater number and severity of exacerbations.15 This has led some authors to suggest the possible presence of a special group of patients with multiple exacerbations that would comprise the frequent exacerbator phenotype.2,4,24,25 In this sense, Chalmers et al. very recently assessed, in the sole existing study on “the frequent exacerbator phenotype” in bronchiectasis, the repeatability of exacerbation status and the independent impact of exacerbation history on hospitalizations, quality of life, and mortality. They concluded that this clinical phenotype has been consistent over time, with a higher mortality risk, and that the best predictor of future exacerbations is previous exacerbations.25
Multidimensional score systems in bronchiectasis, despite demonstrating an excellent predictive capacity for short- and long-term mortality,11–15,28,29 were constructed with variables recorded close to the diagnosis of bronchiectasis and they therefore fail to take into account the number or severity of the exacerbations that occur during the follow-up period. This suggests that, as it is possible that patients who initially present a more severe form of the disease may go on to present a greater number or severity of exacerbations, a high annual incidence of exacerbations over a prolonged period of time could provide additional prognostic information not supplied by the scores of severity.
This study has produced various interesting findings. One the one hand, its conclusions confirm the findings of the Chalmers’ study on the particular relevance of patients with bronchiectasis and multiple exacerbations, even in a very different geographical setting. Moreover, we found that this prognostic value is independent of the initial severity of bronchiectasis, so both variables have an additional prognostic value of all-cause mortality after 5 years of follow-up. Of all the different possible definitions of the “frequent exacerbator patient” analyzed, the one corresponding to the presence of at least two exacerbations per year or at least one hospitalization per year presented, according to our results, the best prognostic capacity of mortality, regardless of the patient's initial severity. It should be stressed that almost all the definitions that included severe exacerbations (hospitalizations) generally showed the greatest predictive power.11,15,25 This result is hardly surprising as several studies have shown that severe exacerbations (hospitalizations) have the most impact on various parameters related to a poor prognosis in bronchiectasis patients, such as a sharper decline in pulmonary function and greater mortality.11–19
On the other hand, those patients described as “exacerbators” over the course of their disease present a different initial clinical picture, a greater initial severity of bronchiectasis, a different microbiological pattern in the follow-up (more microbiological isolates in the stable phase), and a different treatment (a greater amount of inhaled antibiotics and macrolides). Finally, they presented 1.8–3.1 times greater probability of death in the follow-up, regardless of the initial severity of the disease.
Our study does have a number of limitations. On the one hand, an exacerbation was defined as a worsening of respiratory symptoms concomitant with the presence of bronchiectasis that required antibiotic treatment. Although this definition made the researchers’ data collection more objective, there is a possible selection bias in favor of more severe forms of the disease. A consensus definition of exacerbation in bronchiectasis for clinical research was recently reached, unfortunately after the beginning of this study.30. In the present study, we have used the exacerbations arising after the radiological diagnosis of bronchiectasis, rather than those arising beforehand, for our data analysis. This was because it was at the point of their diagnosis that the patients started to be monitored in specialist bronchiectasis units, and so the register of exacerbations over time became much more reliable.
On the other hand, the exacerbation rate observed in the Latin American series is lower than that in other countries as well as the etiologies of bronchiectasis are different (probably in Latin America the percentage of post-tuberculous bronchiectasis is higher than that seen in Europe), and so an external validation of our results is required in other countries with higher rates and patients with different characteristics especially related to different etiologies and age. The lower rate observed in our study could have various causes such as reduced access to medical care in Latin America and a more limited perception of exacerbations by patients, who only consult a doctor when their clinical situation is severe. Factors such as these lead to great geographical heterogeneity with respect to the annual rate of exacerbations reported in different parts of the world. For example, one study of seven European cohorts of bronchiectasis patients from different countries found a rate of one exacerbation/year in Serbia (similar to the rate observed in our Latin American series) but up to 3.4 exacerbations/year in Newcastle-upon-Tyne, UK.12 Finally, we had no information about the stability of the yearly exacerbation rate during follow-up, however some authors have recently reported that exacerbation frequency showed relative stability over time in bronchiectasis patients with multiple exacerbations.25 It would therefore be interesting to conduct further studies in order to ascertain the prognostic capacity of the presence of multiple exacerbations over shorter time periods.
In conclusion, our results show that a certain percentage of bronchiectasis patients (called “frequent exacerbator patients”) are characterized by a high annual rate of exacerbations, with their own clinical characteristics and a poorer prognosis. Moreover, this prognostic value is independent of the initial severity of bronchiectasis, and the combination of at least two exacerbations per year or one hospitalization per year seems to have the best predictive value of mortality in our series. It is important to identify bronchiectasis patients with multiple exacerbations as they could benefit from a more personalized treatment and more specific research studies. In any case, more wide-ranging studies are needed for the external validation of our results in countries with different annual exacerbation rates, as well as other studies investigating whether the presence of multiple exacerbations is associated with an endotype that would explain this high frequency of annual exacerbations in these patients, despite the treatment that they receive.
Conflict of InterestsThe authors declare that they have no conflict of interests.