The Smoking and the Diffuse Interstitial Lung Diseases (ILD) groups of ALAT and SEPAR collaborated in the preparation of this document.
Materials and methodsThis document uses PICO methodology to answer various questions on the relationship between tobacco use and diffuse ILD.
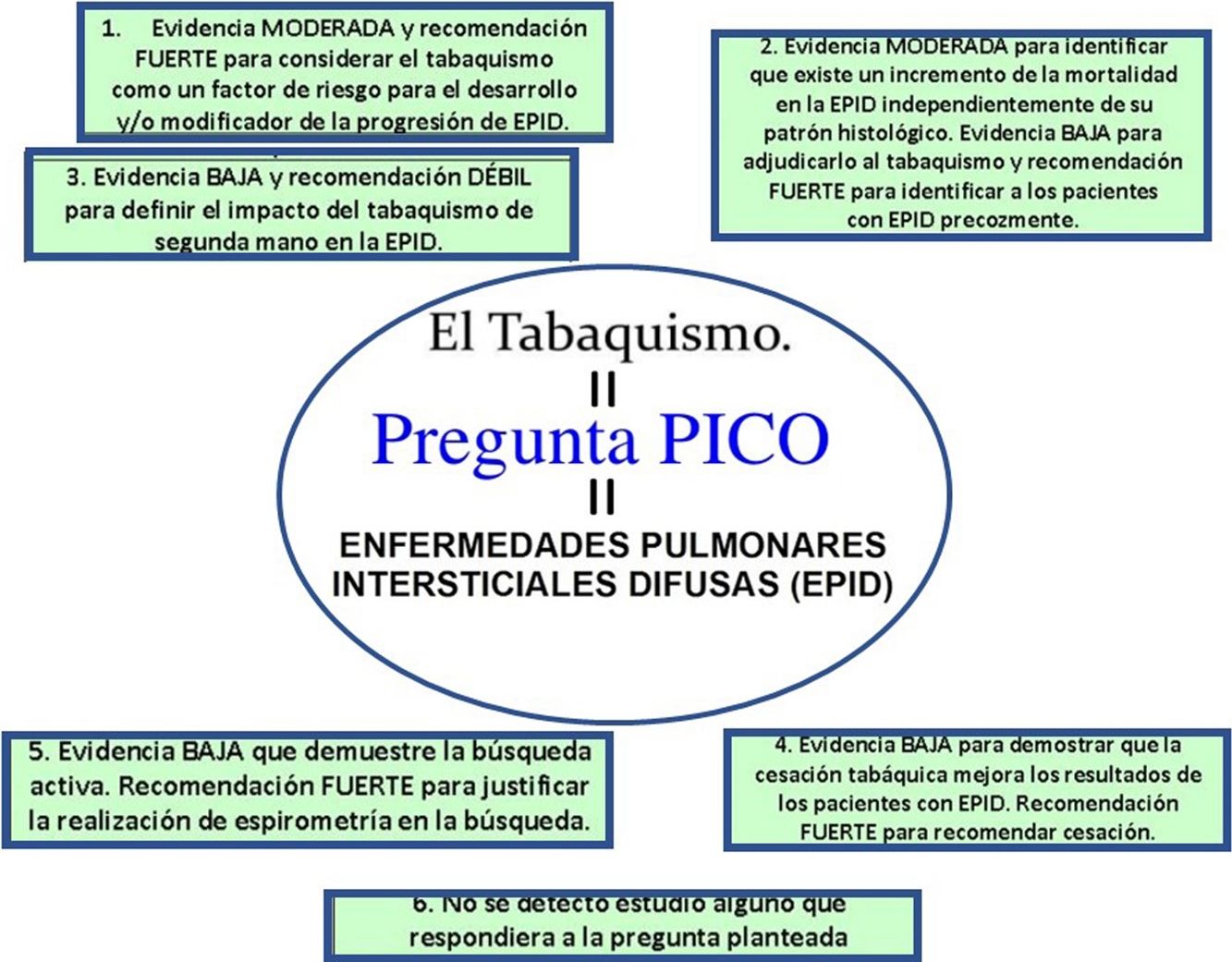
Results and conclusionsThe main recommendations are: (a) moderate level of evidence and strong recommendation to consider smoking as a risk factor for the development and/or modification of the progression of diffuse ILD; (b) moderate level of evidence to identify an increase in mortality in diffuse ILD, irrespective of histologic pattern. Low evidence for ascribing it to smoking and strong recommendation for the early identification of patients with diffuse ILD. Further studies are needed to evaluate the effect of smoking cessation in patients with diffuse ILD; (c) low level of evidence and weak recommendation for defining the impact of passive smoking in diffuse ILD; (d) low level of evidence to demonstrate that smoking cessation improves the outcomes of patients diagnosed with diffuse ILD and strong recommendation to advise smoking cessation in smokers with diffuse ILD, and (e) low level of evidence to support the clinical or epidemiological usefulness of active case finding for diffuse ILD in smoking cessation programs, and strong recommendation justifying the performance of spirometry in active case finding, based not on current smoking status, but on previous accumulated consumption, even in asymptomatic cases.
Los grupos de Tabaquismo y de Enfermedades Pulmonares Intersticiales Difusas (EPID) de ALAT y SEPAR han colaborado para la realización de este documento.
Material y métodosEn el mismo se da respuesta, siguiendo metodología PICO, a diferentes interrogantes sobre la relación entre el consumo de tabaco y las EPID.
Resultados y conclusionesSus principales recomendaciones son: (a) evidencia moderada y recomendación fuerte para considerar el tabaquismo como un factor de riesgo para el desarrollo y/o modificador de la progresión de EPID; (b) evidencia moderada para identificar que existe un incremento de la mortalidad en la EPID independientemente de su patrón histológico. Evidencia baja para adjudicarlo al tabaquismo y recomendación fuerte para identificar a los pacientes con EPID precozmente. Se hacen necesarios nuevos estudios que evalúen el efecto de la cesación tabáquica en los pacientes con EPID; (c) evidencia baja y recomendación débil para definir el impacto del tabaquismo de segunda mano en la EPID; (d) evidencia baja para demostrar que la cesación tabáquica mejora los resultados de los pacientes diagnosticados de EPID y recomendación fuerte para aconsejar la cesación tabáquica en casos de EPID en fumadores, y (e) evidencia baja que demuestre la utilidad clínica o epidemiológica de la búsqueda activa de los casos de EPID en los programas de cesación tabáquica y recomendación fuerte para justificar la realización de espirometría durante esta búsqueda independientemente del estatus actual de tabaquismo pero con la dosis acumulada previamente, aun en casos asintomáticos.
Smoking is a factor involved in a heterogeneous group of diffuse interstitial lung diseases (ILD), although the association ranges from a causal relationship in respiratory bronchiolitis-interstitial lung disease, desquamative interstitial pneumonia, and pulmonary Langerhans cell histiocytosis, to situations in which tobacco acts as a cofactor, such as idiopathic pulmonary fibrosis (IPF) and connective tissue disease-related interstitial diseases.1 This review addresses a series of current issues associated with smoking and ILD.
The Smoking and Interstitial Disease Control and Treatment Groups of the Argentine Association of Respiratory Medicine (AAMR), the Latin American Thoracic Society (ALAT) and the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) have collaborated in this document which aims to respond to various clinical questions using PICO methodology, focusing on 2 aspects (smoking and interstitial disease) of this complex and heterogeneous healthcare problem.
MethodologyFormation of the Collaborative Group and Formulation of Clinical QuestionsAn in-person meeting was held in June 2018, during which the methodology was discussed and clinical scenarios were selected to address the questions to be formulated. A comprehensive search was first conducted to define the feasibility of answering, on the basis of scientific evidence, the questions initially raised; the final questions to be answered were subsequently discussed and agreed on in teleconferences and by emails (Table 1). Working pairs consisting of experts in smoking and interstitial disease were formed with the participation of members from the 3 participating medical societies.
MeSh Terms for the Extended Search of PICO Questions.
| (1) Smoking and ILD: 47 results (October 2018) |
| Search (“Tobacco Smoke Pollution” [Mesh] OR pollution, tobacco smoke OR pollutions, tobacco smoke OR smoke pollution, tobacco OR smoke pollution, tobacco OR tobacco smoke pollutions OR environmental tobacco smoke pollution OR environmental smoke pollution, tobacco OR air pollution, tobacco smoke OR environmental pollution, tobacco smoke OR smoking, passive smoking OR smoking, passive OR second hand smoking OR second hand smoking OR smoking, second hand OR smoking, second hand second hand smoke OR hand smoke, second OR hand smoke, second OR second hand smoke OR smoke, second hand OR smokes, second hand second hand smoke OR second hand smoke OR smoke, second hand OR smokes, second hand OR involuntary smoking OR involuntary smoking OR smoking, involuntary OR smoking, involuntary OR passive smoking)) AND (“Lung Diseases, Interstitial” [Mesh] OR Lung Disease, Interstitial OR Interstitial Lung Diseases OR Diffuse Parenchymal Lung Diseases OR Pneumonia, Interstitial OR Interstitial Pneumonia OR Interstitial Pneumonias OR Pneumonias, Interstitial OR Pneumonitis, Interstitial OR Interstitial Pneumonitides OR Interstitial Pneumonitis OR Pneumonitides, Interstitial) Sort by: Best Match |
| (2) ILD and smoking cessation: 176 results (October 2018) |
| Search (“Lung Diseases, Interstitial” [Mesh] OR Lung Disease, Interstitial OR Interstitial Lung Diseases OR Diffuse Parenchymal Lung Diseases OR Pneumonia, Interstitial OR Interstitial Pneumonia OR Interstitial Pneumonias OR Pneumonias, Interstitial OR Pneumonitis, Interstitial OR Interstitial Pneumonitides OR Interstitial Pneumonitis OR Pneumonitides, Interstitial)) AND (((cessation, smoking OR cessations, smoking OR smoking cessation) OR “Smoking Cessation” [Mesh]) |
In order to focus the search for available evidence, all clinical questions were formulated according to the PICO format or the PECO variant: Patient (Problem or Population), Intervention or Exposure, Comparison and Outcome (relevant outcome).2
The literature search strategy was conducted simultaneously in 2 metasearch engines, Tripddatabase and PubMed using MeSh (Table 1). A 2-way approach was adopted, addressing the importance of tobacco in diffuse ILDs and the impact of smoking cessation on diffuse ILDs.
Eligibility CriteriaThe results retrieved for PICO questions were prioritized according to the highest level of evidence and the most appropriate answer to the clinical question. If this was not possible, intermediate or low level studies in the hierarchy of evidence were selected. The recommended algorithm selection method was used primarily for therapeutic questions.3 Studies published in Spanish, Portuguese and English were considered for inclusion. The end date of the search was October 2018. Table 2 summarizes the search results and the type of study selected to answer the PICO questions.
Number and Type of Studies Selected to Answer the Clinical Questions.
| Clinical Question | Number of References to Answer the Question and Selected Study Type |
|---|---|
| Is smoking a risk factor for the development or progression of ILD? | 1 case-control study73 cohort studies8–10 |
| Does ILD mortality increase in smokers? | 5 cohort studies11–15 |
| What is the importance of second-hand smoking in ILD? | 1 observational study17 |
| Can smoking cessation improve the outcome of patients diagnosed with ILD? | 1 case-control study221 cohort study23 |
| Is active case finding for ILD in smoking cessation programs justified? | 1 cohort study24 |
| Does active smoking affect the efficacy and safety of antifibrotic drugs used in patients with IPF? | NA |
Recommendations and templates developed by the CASPE network (www.redcaspe.org) were used for the critical appraisal of the selected references. The ACCP grading system was used to classify recommendations as STRONG1 or WEAK2 according to the balance of risk, benefits, burdens, and, in some cases, cost. The quality of evidence was classified as HIGH (A), MODERATE (B) or LOW (C), depending on the study design, consistency of results, and ability of the evidence to clearly answer the PICO question.4
The authors proposed a group of external reviewers with experience in the field of diffuse ILDs and smoking, who are listed in the “Authors and collaborators” section.
Scope and ObjectivesThe main objective of this document is to provide all health professionals in general and those working in the areas of smoking and diffuse ILD in particular with up-to-date scientific information on several clinical questions relevant to the management of smokers with ILD and the diagnosis of ILD in tobacco cessation programs.
List of PICO QuestionsQuestion 1: Is Smoking a Risk Factor for the Development or Progression of ILD?RationaleSmoking is a global epidemic that causes the death of nearly 7 million people every year.5 There is strong evidence of the relationship between smoking and the development of cardiovascular and respiratory diseases and cancer.6,7 In recent years, cohort studies of patients with chronic obstructive pulmonary disease (COPD) have been published, showing an increased prevalence of interstitial lung abnormalities (ILA)1 which may reflect a potential causal relationship between smoking and the development of ILD.8 However, these studies often fail to take confounding variables into account. Smoking is also a risk factor for the development of certain autoimmune diseases, many of which may present with ILD, although it is not clear whether there is an independent relationship between smoking and the development of ILD in that setting.1 We must therefore determine the causal relationship between smoking and the development of ILD.
Search SelectionFour studies were included: 1 case-control study9 and 3 cohort studies.10–12
Summary of EvidenceA case-control study aimed at assessing the association between COPD and the presence of ILA9 included patients from 2 studies, one on lung cancer screening (MILD trial)10 and the other on computed tomographic findings in a population of COPD patients.11 Overall, 457 patients (cases) with COPD and 914 patients (controls) without obstruction were selected. The images were evaluated by 2 independent radiologists. Logistic regression analysis showed that current smoking (OR: 4.05; 95% CI: 2.2–7.4) and a higher smoking burden (OR: 1.01; 95% CI: 1–1.02) were factors associated with the presence of definite ILAs. Subjects with fibrotic ILA were more frequently men (OR: 8.58; 95% CI: 1.58–68.9), and they were older (OR 1.17; 95% CI: 1.58–68.9). Airflow obstruction was not significantly associated with definite ILAs or fibrotic ILA.9
A 9-year retrospective longitudinal population study evaluated the risk factors associated with the development of ILD in a group of patients over 40 years of age. Overall, 312519 subjects were analyzed, of which 1972 developed ILD during the study period, with an incidence of ILD of 70.1 cases per 100000 individuals/year. The presence of ILD was more frequent in men and the risk of developing ILD increased with age. Finally, a multivariate analysis showed that smoking was significantly associated with the development of ILD (HR: 1.2; 95% CI: 1.1–1.4).12
Some studies show that smoking increases the risk of developing autoimmune diseases,8 many of which present as interstitial lung involvement. Caucasian smokers with a diagnosis of inflammatory myopathies were more likely to have ILD than non-smokers (OR: 1.52; 95% CI: 1.002–2.29; P=.049) and the study population had a 2% higher chance of presenting ILD for each pack/year (OR: 1.02; 95% CI: 1.004–1.03; P=.010).13
Finally, the influence of smoking was retrospectively analyzed in a small series of 31 patients with non-specific interstitial pneumonia, 16 of whom had non-specific interstitial pneumonia and 15 non-specific connective tissue disease-related interstitial pneumonia. Smoking negatively affected both clinical and radiological disease progression (P=.0489), and reduced the alveolar volume-adjusted carbon monoxide diffusion capacity (DLco/VA), although the limitations of the study prevented the determination of prognosis or causality.14
Conclusions and RecommendationsMODERATE evidence and STRONG recommendation to consider smoking as a risk factor for the development and/or modification of ILD progression.
Recommendation 1BQuestion 2: Does ILD Mortality Increase in Smokers?RationaleSmoking is the leading preventable cause of mortality and leads to premature death in 50% of smokers.5 It is therefore important to determine whether there is an association in terms of mortality between ILD and smoking.
Search SelectionWe included 5 cohort studies.15–19
Summary of EvidenceThe results of large population cohort studies such as the Framingham Heart Study,20 the Age Gene/Environment Susceptibility-Reykjavik study 21, the COPDGene cohort22,23 and ECLIPSE24 found that ILA was associated with an increased risk of all-cause mortality over an average follow-up period of 3 to 9 years. Although there were some discrepancies in the data from 2 cohorts,20,21 the high risk of mortality associated with ILAs among non-smokers in the Framingham Heart Study was due to the small number of deaths in this group.16
A prospective, randomized, controlled lung cancer screening trial was conducted using computed tomography (CT), which included 4101 men and women aged 50 to 70 years, with a history of smoking of at least 20 pack-years.25 Subsequently,15 1,920 patients from this study were included in a second analysis to investigate whether the incidental finding of ILA observed in this cohort was associated with an increase in mortality over a 12-year follow-up. In total, 16.7% of these “healthy” smokers had interstitial involvement and higher all-cause mortality than patients without interstitial involvement, irrespective of their ILD histologic pattern. The findings were associated with death from both lung cancer (HR: 3.2; 95% CI: 1.7–6.2; P<.001) and non-pulmonary malignancy (HR: 2.1; 95% CI: 1.1–4; P=.02).15
In a recently published phenotypic cluster analysis, elderly male smokers with honeycombing showed the worst survival.17 Additionally, among the 8266 patients included in the COPDGene cohort, the combination of ILD and emphysema in smokers was associated with increased clinical and functional severity and an 82% increase in mortality (P<.01) compared to those with emphysema alone.18
In another study that analyzed the patterns of interstitial involvement and mortality in rheumatoid arthritis, smoking was not associated with higher mortality; however, 63% of the patients included were smokers or former smokers, meaning that smoking was a factor associated with higher prevalence of rheumatoid arthritis and interstitial involvement. Additionally, the risk of mortality was higher among patients with DLco<40% predicted (HR: 2.48; 95% CI: 1.55–3.95). Sex, smoking and COPD were not associated with mortality.19
Conclusions and RecommendationsMODERATE evidence to identify an increase in mortality in ILD irrespective of histologic pattern. LOW evidence attributing it to smoking and STRONG recommendation for the early identification of patients with ILD.
Further studies are needed to assess the effect of smoking cessation in patients with ILD.
Recommendation 1BQuestion 3: What is the Importance of Second-hand Smoking in ILD?RationalePassive smoking causes serious malignant, cardiovascular and respiratory diseases. This damage is directly related to the intensity and time of exposure.26 The existence of a relationship between second-hand smoking and the likelihood of developing ILD should be determined.
Summary of EvidenceA multicenter cross-sectional study that analyzed the relationship between second-hand smoking and the finding of various interstitial alterations in high-resolution chest computed tomography (CT) in workers exposed to asbestos was evaluated.27 Exposure to second-hand smoke was assessed using questionnaires. The results showed that involuntary exposure to tobacco smoke was accompanied by an increase in ground glass opacities on high-resolution chest CT. There was also a relationship between lifetime exposure and the presence of linear opacities, although the difference did not reach statistical significance. When exposure to second-hand tobacco smoke in the home over the past 12 months was analyzed separately, a statistically significant relationship was found with the presence of irregular linear opacities (OR: 1.873; 95% CI: 0.512–3.23; P=.007) and with images of honeycombing (OR: 1.10; 95% CI: 0.675–1.524; P=.0001). However, this study had some limitations.27
Conclusions and RecommendationsLOW evidence and WEAK recommendation to define the impact of second-hand smoking on ILD.
Recommendation 2CQuestion 4: Can Smoking Cessation Improve the Outcome of Patients Diagnosed With ILD?RationaleIn some ILDs, smoking is considered the main pathogenic factor.1 A new entity, smoking-related interstitial fibrosis (SRIF) that appears almost exclusively in heavy smokers, has been described.28 In IPF and interstitial involvement in patients with rheumatoid arthritis, smoking appears to act in synergy with genetic factors.1,8 In the absence of specific treatment with proven efficacy, smoking cessation is the main recommendation for the cure or improvement of tobacco-related interstitial diseases. Radiological stabilization or even improvement of lung function after smoking cessation has been described in patients with pulmonary Langerhans cell histiocytosis.29,30 In contrast, continuing to smoke seems to have a negative effect on the progression of interstitial diseases.31 Moreover, smokers with interstitial disease have an increased risk of developing lung cancer.12 Therefore, it seems logical that stopping smoking can improve health outcomes and improve prognosis in patients with ILD.
Search SelectionTwo studies were included: 1 case-control study32 and 1 cohort study.33
Summary of EvidenceNakanishi et al.32 retrospectively studied changes in the radiological and functional findings of 5 patients with respiratory bronchiolitis-associated ILD diagnosed by surgical biopsy after smoking cessation as the only treatment. None of them received steroid treatment or any other immunosuppressive therapy. Previous tobacco use in patients was 30, 32, 40, 28 and 56 pack-years respectively. All patients stopped smoking after diagnosis. Follow-up continued for 15–62 months (46.4±20.8 months). Radiologic changes were analyzed using a semiquantitative visual method. Symptoms improved in all patients after smoking cessation. There was an improvement in CO diffusing capacity (DLco) and oxygenation. There was also an improvement in radiological changes in all cases. The authors concluded that functional and radiological improvement occurs after a follow-up of 46.4±20.8 months as a result of only stopping smoking.
Fabre et al.33 prospectively analyzed the presence of SRIF in 20 patients undergoing lobectomy for lung cancer. All patients were smokers. They were compared with a retrospective group of SRIF cases obtained from the histological study of patients undergoing lung transplantation and with the results of the published studies on SRIF. In the first group, 40% (8/20) had SRIF: these patients were more likely to be smokers than non-SRIF patients (P=.02). In the group of transplanted patients, SRIF was observed in 10% of patients with IPF and in 35% of patients with COPD. From the analysis of all pooled cases, the authors found that patients with SRIF were 15.05 years older than non-SRIF cases, were more likely to be former smokers and have less radiological abnormalities consistent with respiratory bronchiolitis, indicating that smoking cessation can reverse radiological changes derived from smoking.
Conclusions and RecommendationsLOW evidence to demonstrate that smoking cessation improves the outcomes of patients diagnosed with ILD. Studies to date have not been designed to assess the objective stated in the PICO question.
STRONG recommendation to advise smoking cessation in smokers with ILD.
Recommendation 1CQuestion 5: Is Active Case Finding for ILD in Smoking Cessation Programs Justified?RationaleThe existence of ILD associated exclusively with tobacco use,18 the knowledge that smoking is an important risk factor for the development of other ILDs,1 and evidence that smoking may confer a poor prognosis in the course of diseases such as IPF34 or ILD associated with rheumatoid arthritis1,35,36 could justify active case finding for ILD in smoking cessation programs.
Search SelectionOne cohort study was included.37
Summary of EvidenceLederer et al.37 conducted the MESA-Lung study to determine whether tobacco use was associated with subclinical parenchymal lung diseases determined by restriction on spirometry and high attenuation areas on CT. They reported a 10% restriction on spirometry and found that this restriction increased by 8% for every 10 pack-years. They also found that the mean volume of high attenuation was 119 cm3 and that this increased at a rate of 1.6cm3 for every 10 pack-years. The authors concluded that active smoking and previous cumulative tobacco use (in pack-years) was independently associated with restriction on spirometry and increased lung attenuation.
Conclusions and RecommendationsLOW evidence showing the clinical or epidemiological utility of active ILD case finding in smoking cessation programs. STRONG recommendation to justify spirometry during active ILD case finding, based not on current smoking status, but on previous accumulated consumption, even in asymptomatic cases.
Recommendation 1CQuestion 6: Does Active Smoking Affect the Efficacy and Safety of Antifibrotic Drugs Used in Patients With IPF?We did not find any study that specifically addressed the interaction of smoking with pirfenidone and nintedanib efficacy in the treatment of IPF. Taking into account the similar efficacy demonstrated by both drugs in lung function and mortality, the choice of treatment in smokers with IPF will be individualized according to their safety profile, contraindications and drug interactions.38,39
FundingThis article has been made possible thanks to an unconditional grant BIESA.
Conflict of InterestAll authors declare that they have no conflict of interest in relation to the article submitted for publication. Dr. Efrain Sánchez Angarita states that he received honoraria for his collaboration with ITSalud/Medsolid. Dr. Agustín Acuña Izcaray states that he has received honoraria for his collaboration with ITSalud/Medsolid.
We express our gratitude to Dr. Angela Ramos Pinedo (Hospital Fundación Alcorcón, Madrid, Spain) and Dr. Eva de Higes Martínez (Hospital Fundación Alcorcón, Madrid, Spain), who were the external reviewers of this document.
Please cite this article as: Jiménez-Ruiz CA, Zabert G, Buljubasich D, de Granda Orive JI, Buendía I, Luhning S, et al. Preguntas y respuestas relacionadas con tabaquismo en pacientes con EPID. Aplicación de metodología con formato PICO listado de autores. Arch Bronconeumol. 2020;56:435–440.