Obesity-hypoventilation syndrome (OHS) is a risk factor for heart failure (HF). Some studies associate the use of non-invasive ventilation (NIV) with changes in hemodynamic parameters. Our objective was to describe the hemodynamic status of a group of patients with OHS and to study the effect of NIV.
Patients and methodsPatients with stable OHS treated with NIV were included in this cross-sectional repeated measurements study. Hemodynamics were measured by bioimpedance: 30min at baseline and another 30min on NIV. Cardiac output (CO), cardiac index, and systolic volume were measured. The CO calculated for each patient expressed as a percentage of the lower limit of normal (LLN) was taken as reference, and 2 groups were formed: patients without HF and normal CO (≥100% of LLN) and patients with HF and low CO (<100% of LLN). The Mann–Whitney U test was used to compare independent variables and the Wilcoxon test was used for paired variables, with significance set at P<.05.
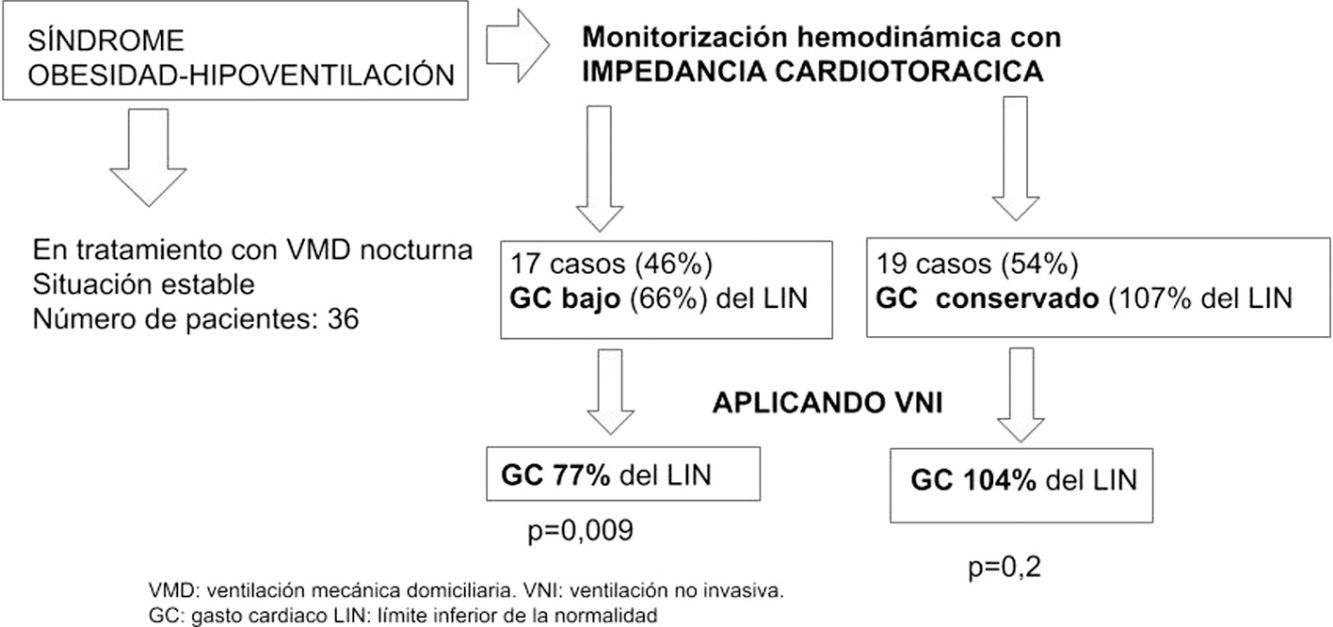
ResultsThe final sample comprised 36 patients, aged 66 (±8) years, 19 (52%) men. In 17 (46%) patients, HF was detected with a CO of 3.7l/min (66%) compared to the group without HF, whose CO was 7l/min (107%). After NIV, patients with HF showed improvement in CO 4.5l/min (77%), P=.009, while the non-HF group remained unchanged, with CO 6.8l/min (104%), P=.2.
ConclusionA total of 46% of patients with stable OHS present HF; NIV improves hemodynamics and does not affect patients with normal CO.
El síndrome de obesidad-hipoventilación (SOH) es un factor de riesgo para presentar insuficiencia cardiaca (IC). Algunos estudios relacionan el uso de ventilación no invasiva (VNI) con la alteración de parámetros hemodinámicos. Nuestro objetivo fue describir la situación hemodinámica de un grupo de pacientes con SOH y estudiar el efecto de la VNI.
Pacientes y métodosCon un diseño transversal de mediciones repetidas, se incluyó a pacientes con SOH tratados con VNI en situación estable. Se estudió su hemodinámica con bioimpedancia: 30min basales y otros 30 con VNI. Se midieron el gasto cardiaco (GC), el índice cardiaco y el volumen sistólico. Se tomó como referencia el GC calculado para cada paciente expresado como porcentaje sobre su límite inferior de normalidad (LIN) y se establecieron 2 grupos: sin IC con GC conservado (≥100% sobre LIN) y con IC y GC bajo (<100% del LIN). Se utilizaron la U de Mann–Whitney para comparación de variables independientes y el test de Wilcoxon para variables apareadas, se consideró significativo p<0,05.
ResultadosLa muestra final fue de 36, de 66 (8) años, 19 (52%) varones. En 17 (46%) detectamos IC con GC de 3,7l/min (66%) frente al grupo sin IC con 7l/min (107%). Tras VNI, los pacientes en IC mejoraron su GC (4,5l/min [77%] p=0,009) y no lo modificó el grupo sin IC: 6,8l/min (104%), p=0,2.
ConclusiónUn 46% de pacientes con SOH estable presenta IC. La VNI mejora su hemodinámica y no influye en los pacientes con GC conservado.
Obesity-hypoventilation syndrome (OHS) is a combination of daytime hypercapnia and obesity in the absence of other causes of hypoventilation. Pathogenesis is multifactorial, and often associated with sleep apnea–hypopnea syndrome (SAHS). It is a risk factor for heart failure (HF)1,2 and various abnormalities in cardiac morphology and function.3,4
Although HF is diagnosed in clinical practice on the basis of clinical, radiological, analytical and echocardiographic criteria, it in fact reflects a situation that involves inadequate cardiac output (CO) and increased pressures in the heart cavities1; these 2 variables constitute the hemodynamic expression of HF and are probably the most objective way of measuring it. Individuals with OHS often receive treatment with positive pressure devices that can affect their hemodynamic status,5 but experience is limited and sometimes contradictory6,7; although the co-existence of HF in patients with OHS2,8,9 is well known, there is no established consensus for the evaluation and management of this situation in clinical practice guidelines.10,11
Cardiothoracic impedance (CTI) provides a non-invasive estimation of hemodynamic data. It consists of the application of a low frequency alternating current to detect changes in volume and blood flow in the chest. The analysis of these flows has helped validate this technique and provides reliable parameters such as: CO, cardiac index (CI), systolic volume (SV), etc.12–16
Our hypothesis is that CTI can be used to explore the hemodynamics of patients with OHS in a non-invasive manner. Our primary objective was to describe the baseline hemodynamic situation in patients with OHS (as a marker for HF) and secondarily to assess the effect that non-invasive ventilation might have on these parameters.
Patients and MethodsPatientsWe included consecutive patients with OHS, body mass index (BMI)>30kg/m2, daytime pCO2>46mmHg, and associated night-time ventilatory disorder (hypoventilation or SAHS) receiving non-invasive ventilation (NIV) at home for at least 6 months, in a stable clinical situation, who came to our clinics for follow-up appointments. Data obtained included age, sex, BMI, lung function: FEV1, FEV1/FVC, DLCO, RV, RV/TLC, baseline pCO2, ventilator parameters (IPAP, EPAP, PS, and respiratory rate), respiratory polygraphy data: apnea–hypopnea index (AHI) and percentage of time with less than 90% SpO2 (T90). Transthoracic echocardiography was performed with measurement of left ventricular ejection fraction (LVEF) and pulmonary artery pressure (PAP); LVEF greater than 50% was considered normal, and PAP equal to or greater than 40mmHg indicated pulmonary hypertension.
Patients included in the study had a diagnosis of OHS and were receiving NIV at home for at least 6 months; NIV was indicated in patients with respiratory failure with daytime hypercapnia and AHI less than 30/h; cases with an AHI greater than 30/h had initially been treated with nasal CPAP, which was replaced by NIV if hypercapnia persisted. VPAP S9 or Lumis (ResMed, Australia) ventilators were used in ST mode. Adaptation was performed in most cases on an outpatient basis, titrating IPAP, EPAP and respiratory rate to obtain comfort, daytime normocapnia, nocturnal SpO2 with T90 less than 30%, and minimal residual events detected on ventilator readouts.
Comorbidity data were obtained from the medical history: arterial hypertension, diabetes and, retrospectively, any diagnosed episodes of decompensated heart failure.
Patients were excluded if they had pacemakers, exacerbations in the previous 2 months, or poor quality CTI studies that could not be appropriately interpreted (stable values not reached during the test or good quality signal not obtained for at least 2 consecutive minutes in each period).
The study was approved by the hospital ethics and research committee and informed consent was obtained from all patients during recruitment.
MethodsPatients were consecutively included using a cross-sectional and observational repeated measures design. Hemodynamic monitoring was performed during the inclusion visit using NICCOMO bioimpedance equipment (Medis Medizinische Messtechnik GmbH, Ilmenau, Germany), providing a continuous (beat-to-beat) non-invasive measurement of cardiac function. Two pairs of specific electrodes were placed on the laterocervical and flank regions above the midaxillary line. The procedure took place with the patient reclining at 45° for a period of 60min, the first 30min at baseline and then another 30min receiving NIV with ventilatory parameters prescribed for the patient for home treatment. Hemodynamic data collected were: heart rate (HR), mean arterial pressure (MAP), CO, CI, SV, and mean SpO2; these variables were then analyzed with specific software (Cardiovascular Lab®, Ilmenau, Germany) provided by the manufacturer.
CO was taken as a reference parameter for the diagnosis of heart failure and the value obtained was expressed as a percentage of the lower limit of normal (LLN) calculated for each patient using the Cardiovascular Lab® software, based on age, sex, and anthropometric data. HF was defined as CO below the LLN. Two groups were established for the analysis of results: those with normal CO (CO ≥100% LLN) and those with a decreased CO (CO <100% LLN). To determine the effect of NIV on the patient's hemodynamics, the same parameters were measured after the application of NIV, and compared with baseline measurements.
The estimated sample size was 40 patients with OHS, with a confidence level of 95% and an accuracy of 15%. It was assumed that the prevalence of OHS in the general population is 0.6%6 and that the heart failure rate is unknown (P=0.5).
Values are expressed as mean and ranges for quantitative variables, and as percentages for qualitative variables. Statistical analysis of quantitative variables was performed using the Mann–Whitney U test for comparison of independent variables and the Wilcoxon test for paired variables. The Chi-squared test was used for qualitative variables. A P-value of <0.05 was considered significant.
ResultsA total of 42 patients were studied, 6 of whom were excluded due to poor quality studies. The final sample was 36 cases, 66 years of age (±8), 19 (52%) males. Twenty-four (66%) had high blood pressure, 18 (50%) were diabetic, and 18 (50%) had had at least one previously diagnosed episode of heart failure. Twenty cases (56%) had severe SAHS with an average AHI of 62/h (38–110) and 16 had mild–moderate SAHS with an AHI of 15/h (8–23).
Table 1 shows the general, functional, and hemodynamic characteristics and ventilatory parameters of all patients included in the study. Heart failure was detected in 17 cases (46%), with a mean CO of 3.7l/min (66% LLN).
Comparative Study of Cases With Normal Cardiac Output Versus Those With Reduced Cardiac Output.
| Cardiac Output | Cardiac Output >LLN | P-value | |
|---|---|---|---|
| N=17 | N=19 | ||
| Age (years) | 71 (55–82) | 61 (31–80) | 0.02 |
| BMI (kg/m2) | 40 (31–56) | 47 (33–65) | 0.04 |
| PO2 (mmHg) | 67 (60–86) | 66 (61–78) | 0.9 |
| pCO2 (mmHg) | 41 (34–46) | 43 (36–46) | 0.2 |
| AHI (apneas/h) | 40 (8–92) | 48 (8–110) | 0.42 |
| T90 (%) | 83 (60–96) | 86 (41–99) | 0.6 |
| FEV1 (% predicted) | 77 (47–119) | 67 (43–104) | 0.5 |
| DLCO (% predicted) | 80 (56–114) | 74 (44–112) | 0.9 |
| RV (% predicted) | 139 (65–180) | 118 (69–182) | 0.6 |
| RV/TLC | 49 (32–66) | 48 (37–60) | 0.7 |
| IPAP (cmH2O) | 17 (14–20) | 17 (15–21) | 0.7 |
| IPAP (cmH2O) | 9 (7–13) | 9 (7–13) | 0.7 |
| PS (cmH2O) | 8 (5–12) | 8 (6–12) | 0.7 |
| Frequency (resp/min) | 13 (7–18) | 14 (6–17) | 0.3 |
| LVEF, n (%) | 66 (45–75) | 64 (55–81) | 0.2 |
| Baseline cardiac output (l/min) | 3.7 (2.5–5.5) | 7.1 (4.8–10.2) | 0.001 |
| Baseline cardiac output (% predicted) | 66 (43–71) | 107 (81–123) | 0.001 |
| Cardiac index | 1.8 (1.23–2.27) | 3 (2.10–5.14) | 0.001 |
| Systolic volume (cm3) | 58 (33.6–74.3) | 102 (57.4–140.3) | 0.001 |
| Mean blood pressure (mmHg) | 97 (83–119) | 103 (82–129) | 0.1 |
| SpO2 (%) | 93 (91–98) | 93 (91–96) | 0.6 |
Values expressed as mean and in parentheses minimum and maximum value.
In parentheses: range of the variable.
BMI: body mass index; EPAP: expiratory pressure; IPAP: inspiratory pressure; LLN: lower limit of normal; LVEF: left ventricular ejection fraction; RV: residual volume; PS: pressure support; T90: recording time in respiratory polygraphy with SpO2 <90%. TLC: total lung capacity.
In the HF group (N=17), 11 subjects were women and 6 were men (P=0.024), 9 patients had hypertension (P=0.15), 9 were diabetics (P=0.31), 8 had previous episodes of HF (P=0.62), and 7 had severe SAHS (P=0.1). Mean age was higher and BMI significantly lower than in patients with low CO. There were no differences in lung function, ventilatory parameters, or LVEF obtained by echocardiography. Table 1 shows the comparative analysis between the two groups.
Table 2 shows the hemodynamic changes that occurred in each patient group after the application of NIV. The group of patients with low CO showed a significant improvement in CO, CI, and SV, while those with normal CO did not experience changes.
Progress of Hemodynamic Parameters After NIV in Both Groups.
| CON=17) | CO >LLN (N=19) | |||||
|---|---|---|---|---|---|---|
| Baseline | With NIV | P | Baseline | With NIV | P | |
| Heart rate (beats/min) | 67 (55–92) | 66 (54–82) | 0.4 | 71 (54–91) | 69 (48–89) | 0.11 |
| Mean blood pressure (mmHg) | 98 (83–119) | 95 (84–117) | 0.1 | 103 (82–129) | 100 (81–121) | 0.06 |
| Cardiac output (l/min) | 3.7 (2.5–5.5) | 4.5 (2.7–6.4) | 0.017 | 7.1 (4.8–10.2) | 6.8 (5.4–10.8) | 0.4 |
| Cardiac output (% predicted) | 66 (43–70) | 77 (51–118) | 0.023 | 107 (81–123) | 104 (85–130) | 0.7 |
| Cardiac index | 1.8 (1.2–2.2) | 2.1 (1.4–3.2) | 0.029 | 3.2 (2.1–5.4) | 2.8 (2.0–4.1) | 0.3 |
| Systolic volume (cm3) | 58 (36–74) | 68 (33–101) | 0.02 | 102 (57–170) | 101 (63–143) | 0.9 |
CO: cardiac output; LLN: lower limit of normal; NIV: non-invasive ventilation.
In parentheses: range of the variable.
The echocardiographic study was carried out in 26 cases, while the remaining cases were unevaluable due to poor echocardiographic views and failure to complete all measurements. Mean pulmonary artery pressure was 36mmHg; in 7 (26%) patients, figures were consistent with pulmonary hypertension (>40mmHg), and LVEF was normal in all cases (greater than 50%).
DiscussionAlmost half (46%) of our patients with stable OHS receiving NIV had hemodynamic dysfunction, with low CO consistent with heart failure. The use of NIV improved CO in patients in HF, but it did not significantly affect patients with normal CO.
Obesity is known to be associated with the presence of cardiological comorbidity and increased risk of episodes of heart failure,1,17,18 and cardiac changes have also been described in OHS patients, especially right heart overload and signs of PHT.3,4 Castro-Añon et al.,19 using echocardiography in patients with OHS, detected right ventricular overload in 43% of cases with preserved LVEF, and Corral et al.20 found echocardiographic alterations in more than half of the patients in their series; progress in both studies was favorable with ventilatory treatment. In our series, pulmonary artery pressure could only be determined in 26 cases, of which 27% had figures indicative of PHT.
The most important finding of our study is that we detected HF in a substantial percentage of patients with OHS based on the measurement of CO. Our series consisted of stable patients who attended scheduled appointments; subjects with HF were predominantly women, older, with a lower BMI than the group without HF. We found no significant differences in the existence of previous episodes of HF, lung function, AHI, LVEF measured by echocardiography, pCO2 values, or ventilator parameters. From a hemodynamic point of view, patients with HF had low SV and CI values, while MAP and heart rate were similar between the 2 groups. One hypothesis for this might be that hemodynamic changes are associated with abnormalities in cardiac function18,20 and would not be related to the underlying ventilatory disease.
Two types of HF are distinguished according to echocardiographic findings: HF with normal LVEF (diastolic HF) and HF with diminished LVEF (low contractility).1 In our series, all cases had LVEF >60%, as observed in the study by Corral et al.20 Otair et al.,9 using echocardiography, also detected diastolic dysfunction in 67% of patients with OHS. These data suggest that HF associated with OHS is predominantly diastolic, with preserved left ventricular contractility.
The application of positive airway pressure may alter hemodynamics in patients with lung disease,6,21,22 although there are conflicting results in this regard. Lukacsovits et al.,6 using non-invasive measurements of CO (Finometer-PRO) in COPD patients, showed that in cases where high intensity ventilation with mean IPAP of 27cmH2O was used, greater reductions were observed in CO than with the use of more moderate pressures (17cmH2O). The authors proposed using lower pressures in cases of underlying heart disease. Leontine et al.,7 using echocardiography in patients with COPD, did not detect changes in CO using slightly lower pressures (23cmH2O). In our study of patients with OHS using maximum pressures of around 20cmH2O in patients with normal LVEF, we found that NIV had different effects depending on the patient's underlying hemodynamic situation: all parameters improved in patients with hemodynamic dysfunction, while those with normal CO showed no changes. Although we believe that our study is the first to describe this situation in a series of patients with OHS, Yoshida et al.23 described the case of a patient with OHS and severe SAHS with hypercapnic respiratory failure monitored with cardiothoracic impedance in whom low CO figures improved rapidly and significantly with the application of positive pressure ventilation. Although there is no definitive explanation for this phenomenon, it could be related to the improvement in oxygenation, decrease in preload and postload, a reduction in cardiac transmural pressure, and in pulmonary circulation resistances.23,24
In our study, patients with normal CO had a higher BMI than those with decreased CO. The association between obesity and HF shows that patients with higher BMI have a higher risk of presenting HF17; this result could be explained by the reduced sample size and should be confirmed in larger series.
Our study has its limitations: we could not perform echocardiography in all patients included and the echocardiography and the CTI procedures were not performed at the same time, so there may be differences in the clinical status of the patients between both examinations; moreover, although the number of patients estimated in the sample calculation were included (40), the final figure assessed was slightly lower (36).
We can conclude that including cardiac and hemodynamic examinations in the management of patients with OHS provides useful information that can help better define their clinical status and their therapeutic needs, and assess their progress and indicate complementary treatments. We found that NIV impacts positively on patients with more altered hemodynamics and normal LVEF, but given our limited data, these findings will have to be confirmed in future studies.
Conflict of InterestsThe authors state that they have no conflict of interests.
Please cite this article as: Fernández Álvarez R, Belda Ramirez J, Rubinos Cuadrado G, Buchelli Ramirez H, Fole Vazquez D, Iscar Urrutia M, et al. Síndrome de obesidad-hipoventilación: situación hemodinamica basal e impacto de la ventilación no invasiva. Arch Bronconeumol. 2020;56:441–445.